NEOMYCIN AND POLYMYXIN B SULFATES AND GRAMICIDIN
Neomycin and Polymyxin B Sulfates and Gramicidin Ophthalmic Solution, USP
9691178e-5323-40ce-a8b3-f79e88f02797
HUMAN PRESCRIPTION DRUG LABEL
Feb 4, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
neomycin sulfate, polymyxin b sulfate and gramicidin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Neomycin and polymyxin B sulfates and gramicidin ophthalmic solution, USP is a sterile antimicrobial solution for ophthalmic use.
Each mL contains: Actives: neomycin sulfate, (equivalent to 1.75 mg neomycin base), polymyxin B sulfate equal to 10,000 polymyxin B units, gramicidin, 0.025 mg;Inactives: sodium chloride, alcohol (0.5%), Poloxamer 188, propylene glycol, purified water. Hydrochloric acid and/or ammonium hydroxide may be added to adjust pH (4.7-6.0).
**Preservative:**thimerosal 0.001%.
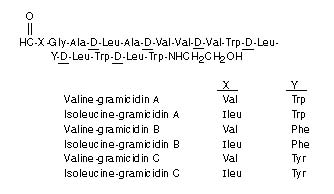
Neomycin Sulfate is the sulfate salt of neomycin B and C, which are produced by the growth of Streptomyces fradiae Waksman (Fam. Streptomycetaceae). It has a potency equivalent of not less than 600 micrograms of neomycin base per milligram, calculated on an anhydrous basis.
The structural formulae are:
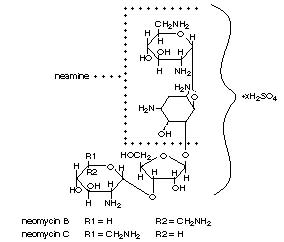
Polymyxin B Sulfate is the sulfate salt of polymyxin B1 and B2 which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units per milligram, calculated on an anhydrous basis. The structural formulae are:
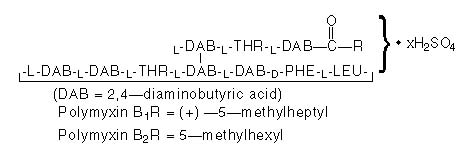
Gramicidin (also called gramicidin D) is a mixture of three pairs of antibacterial substances (Gramicidin A, B and C) produced by the growth of Bacillus brevis Dubos (Fam. Bacillaceae). It has a potency of not less than 900 mcg of standard gramicidin per mg. The structural formulae are: