WEGOVY
These highlights do not include all the information needed to use WEGOVY safely and effectively. See full prescribing information for WEGOVY.WEGOVY (semaglutide) injection, for subcutaneous useInitial U.S. Approval: 2017
ee06186f-2aa3-4990-a760-757579d8f77b
HUMAN PRESCRIPTION DRUG LABEL
Aug 1, 2025
Novo Nordisk
DUNS: 622920320
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
semaglutide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
semaglutide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
semaglutide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
semaglutide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
semaglutide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
wegovy® 2.4 mg
NDC 0169-4524-14 List 452414
2.4 mg
wegovy**®**
(semaglutide) injection
2.4 mg/0.75 mL
Use Wegovy 1 time a week
4 Single-Dose Prefilled Pens
Each pen delivers a single dose of 2.4 mg semaglutide
For subcutaneous use only
Single-Dose only
Rx only
Contains: 4 Wegovy pens, Product Literature.
Dispense the enclosed Medication Guide to each patient.
Boxed Warning section
WARNING: RISK OF THYROID C-CELL TUMORS
See full prescribing information for complete boxed warning.
•
**In rodents, semaglutide causes thyroid C-cell tumors at clinically relevant exposures. It is unknown whether WEGOVY causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as the human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined (****5.1****,****13.1****).**
•
**WEGOVY is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk of MTC and symptoms of thyroid tumors (****4****,****5.1****).**
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
WEGOVY is indicated in combination with a reduced calorie diet and increased physical activity:
•
to reduce the risk of major adverse cardiovascular (CV) events (CV death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established CV disease and either obesity or overweight.
•
to reduce excess body weight and maintain weight reduction long term in:
o
Adults and pediatric patients aged 12 years and older with obesity
o
Adults with overweight in the presence of at least one weight-related comorbid condition.
•
for the treatment of noncirrhotic metabolic dysfunction-associated steatohepatitis (MASH), formerly known as nonalcoholic steatohepatitis (NASH), with moderate to advanced liver fibrosis (consistent with stages F2 to F3 fibrosis) in adults.
The indication for MASH is approved under accelerated approval based on improvement of MASH and fibrosis [see Clinical Studies (14.4)]. Continued approval for this indication may be contingent upon the verification and description of clinical benefit in a confirmatory trial.
Limitations of Use
WEGOVY contains semaglutide. Coadministration with other semaglutide- containing products or with any other GLP-1 receptor agonist is not recommended.
WEGOVY is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated in combination with a reduced calorie diet and increased physical activity:
•
to reduce the risk of major adverse cardiovascular (CV) events (CV death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established CV disease and either obesity or overweight. (1)
•
to reduce excess body weight and maintain weight reduction long term in:
o
Adults and pediatric patients aged 12 years and older with obesity.
o
Adults with overweight in the presence of at least one weight-related comorbid condition. (1)
•
for the treatment of noncirrhotic metabolic dysfunction-associated steatohepatitis (MASH), formerly known as nonalcoholic steatohepatitis (NASH), with moderate to advanced liver fibrosis (consistent with stages F2 to F3 fibrosis) in adults. (1)
The indication for MASH is approved under accelerated approval based on improvement of MASH and fibrosis. Continued approval for this indication may be contingent upon the verification and description of clinical benefit in a confirmatory trial. (1)
Limitations of Use
Coadministration with other semaglutide-containing products or with any other GLP-1 receptor agonist is not recommended. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
WEGOVY is contraindicated in the following conditions:
•
A personal or family history of MTC or in patients with MEN 2 [see Warnings and Precautions (5.1)].
•
A prior serious hypersensitivity reaction to semaglutide or to any of the excipients in WEGOVY. Serious hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with WEGOVY [see Warnings and Precautions (5.7)].
•
Personal or family history of MTC or in patients with MEN2. (4)
•
Known hypersensitivity to semaglutide or any of the excipients in WEGOVY (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Thyroid C-Cell Tumors
In mice and rats, semaglutide caused a dose-dependent and treatment-duration- dependent increase in the incidence of thyroid C-cell tumors (adenomas and carcinomas) after lifetime exposure at clinically relevant plasma exposures [see Nonclinical Toxicology (13.1)]. It is unknown whether WEGOVY causes thyroid C-cell tumors, including MTC, in humans, as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined.
Cases of MTC in patients treated with liraglutide, another GLP-1 receptor agonist, have been reported in the postmarketing period; the data in these reports are insufficient to establish or exclude a causal relationship between MTC and GLP-1 receptor agonist use in humans.
WEGOVY is contraindicated in patients with a personal or family history of MTC or in patients with MEN2. Counsel patients regarding the potential risk for MTC with the use of WEGOVY and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness).
Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with WEGOVY. Such monitoring may increase the risk of unnecessary procedures, due to the low-test specificity for serum calcitonin and a high background incidence of thyroid disease. Significantly elevated serum calcitonin value may indicate MTC and patients with MTC usually have calcitonin values greater than 50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical examination or neck imaging should also be further evaluated.
5.2 Acute Pancreatitis
Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including WEGOVY [see Adverse Reactions (6)]. After initiation of WEGOVY, observe patients carefully for signs and symptoms of acute pancreatitis, which may include persistent or severe abdominal pain (sometimes radiating to the back), and which may or may not be accompanied by nausea or vomiting. If acute pancreatitis is suspected, discontinue WEGOVY and initiate appropriate management.
5.3 Acute Gallbladder Disease
Treatment with WEGOVY is associated with an increased occurrence of cholelithiasis and cholecystitis. The incidence of cholelithiasis and cholecystitis was higher in WEGOVY-treated pediatric patients aged 12 years and older than in WEGOVY-treated adults. In randomized clinical trials in adults for weight reduction, cholelithiasis was reported by 1.6% of WEGOVY- treated patients and 0.7% of placebo-treated patients. Cholecystitis was reported by 0.6% of WEGOVY-treated adult patients and 0.2% of placebo-treated patients. In a clinical trial in pediatric patients aged 12 years and older for weight reduction, cholelithiasis was reported by 3.8% of WEGOVY-treated patients and 0% placebo-treated patients. Cholecystitis was reported by 0.8% of WEGOVY-treated pediatric patients and 0% placebo-treated patients [see Adverse Reactions (6.1)].
Substantial or rapid weight loss can increase the risk of cholelithiasis; however, the incidence of acute gallbladder disease was greater in WEGOVY- treated patients than in placebo-treated patients, even after accounting for the degree of weight loss. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated.
5.4 Hypoglycemia
WEGOVY lowers blood glucose and can cause hypoglycemia.
In a trial of adult patients with type 2 diabetes and body mass index (BMI) greater than or equal to 27 kg/m2 for weight reduction (Study 3), hypoglycemia (defined as a plasma glucose less than 54 mg/dL) was reported in 6.2% of WEGOVY-treated patients versus 2.5% of placebo-treated patients. One episode of severe hypoglycemia (requiring the assistance of another person) was reported in one WEGOVY-treated patient versus no placebo-treated patients [see Clinical Studies (14.2)].
Patients with diabetes mellitus taking WEGOVY in combination with insulin or an insulin secretagogue (e.g., sulfonylurea) may have an increased risk of hypoglycemia, including severe hypoglycemia. Hypoglycemia has been observed in patients treated with semaglutide at doses of 0.5 mg and 1 mg in combination with insulin. The use of WEGOVY (semaglutide 2.4 mg or 1.7 mg once weekly) in patients with type 1 diabetes mellitus or in combination with insulin has not been evaluated.
Inform patients of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia. In patients with diabetes, monitor blood glucose prior to starting WEGOVY and during WEGOVY treatment. When initiating WEGOVY, consider reducing the dose of concomitantly administered insulin or insulin secretagogue (such as sulfonylureas) to reduce the risk of hypoglycemia [see Drug Interactions (7.1)].
5.5 Acute Kidney Injury Due to Volume Depletion
There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of the reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea [see Adverse Reactions (6)].
Monitor renal function in patients reporting adverse reactions to WEGOVY that could lead to volume depletion, especially during dosage initiation and escalation of WEGOVY.
5.6 Severe Gastrointestinal Adverse Reactions
Use of WEGOVY has been associated with gastrointestinal adverse reactions, sometimes severe [seeAdverse Reactions (6.1)]. In WEGOVY clinical trials in adults for weight reduction, severe gastrointestinal adverse reactions were reported more frequently among patients receiving WEGOVY (4.1%) than placebo (0.9%).
WEGOVY is not recommended in patients with severe gastroparesis.
5.7 Hypersensitivity Reactions
Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported with WEGOVY. If hypersensitivity reactions occur, discontinue use of WEGOVY, treat promptly per standard of care, and monitor until signs and symptoms resolve. WEGOVY is contraindicated in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in WEGOVY [see Adverse Reactions (6.2)].
Anaphylaxis and angioedema have been reported with other GLP-1 receptor agonists. Use caution in a patient with a history of anaphylaxis or angioedema with another GLP-1 receptor agonist because it is unknown whether such patients will be predisposed to these reactions with WEGOVY.
5.8 Diabetic Retinopathy Complications in Patients with Type 2 Diabetes
In a 2-year trial with semaglutide 0.5 mg and 1 mg once-weekly injection in adult patients with type 2 diabetes and high CV risk, diabetic retinopathy complications (which was a 4-component adjudicated endpoint) occurred in patients treated with semaglutide injection (3%) compared to placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline (semaglutide injection 8.2%, placebo 5.2%) than among patients without a known history of diabetic retinopathy (semaglutide injection 0.7%, placebo 0.4%).
In a trial of adult patients with type 2 diabetes and BMI greater than or equal to 27 kg/m2 for weight reduction (Study 3), diabetic retinopathy was reported by 4% of WEGOVY-treated patients and 2.7% placebo-treated patients [see Clinical Studies (14.2)].
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. The effect of long-term glycemic control with semaglutide on diabetic retinopathy complications has not been studied. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy.
5.9 Heart Rate Increase
Treatment with WEGOVY was associated with increases in resting heart rate. Mean increases in resting heart rate of 1 to 4 beats per minute (bpm) were observed in WEGOVY-treated adult patients compared to placebo in clinical trials for weight reduction. More adult patients treated with WEGOVY compared with placebo had maximum changes from baseline at any visit of 10 to 19 bpm (41% versus 34%, respectively) and 20 bpm or more (26% versus 16%, respectively). In a clinical trial in pediatric patients aged 12 years and older with normal baseline heart rate, more patients treated with WEGOVY compared to placebo had maximum changes in heart rate of 20 bpm or more (54% versus 39%) [see Adverse Reactions (6.1)].
Monitor heart rate at regular intervals consistent with usual clinical practice. Instruct patients to inform their healthcare providers of palpitations or feelings of a racing heartbeat while at rest during WEGOVY treatment. If patients experience a sustained increase in resting heart rate, discontinue WEGOVY.
5.10 Suicidal Behavior and Ideation
Suicidal behavior and ideation have been reported in clinical trials with other weight management products. Monitor patients treated with WEGOVY for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Discontinue WEGOVY in patients who experience suicidal thoughts or behaviors. Avoid WEGOVY in patients with a history of suicidal attempts or active suicidal ideation.
5.11 Pulmonary Aspiration During General Anesthesia or Deep Sedation
WEGOVY delays gastric emptying [see Clinical Pharmacology(12.2)]. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations.
Available data are insufficient to inform recommendations to mitigate the risk of pulmonary aspiration during general anesthesia or deep sedation in patients taking WEGOVY, including whether modifying preoperative fasting recommendations or temporarily discontinuing WEGOVY could reduce the incidence of retained gastric contents. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking WEGOVY.
•
Acute Pancreatitis: Has been observed in patients treated with GLP-1 receptor agonists, including WEGOVY. Discontinue promptly if pancreatitis is suspected. (5.2)
•
Acute Gallbladder Disease: Has occurred in clinical trials. If cholelithiasis is suspected, gallbladder studies and clinical follow-up are indicated. (5.3)
•
Hypoglycemia: Concomitant use with insulin or an insulin secretagogue may increase the risk of hypoglycemia, including severe hypoglycemia. Reducing the dose of insulin or insulin secretagogue may be necessary. Inform all patients of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia. (5.4)
•
Acute Kidney Injury Due to Volume Depletion: Monitor renal function in patients reporting adverse reactions that could lead to volume depletion. (5.5)
•
Severe Gastrointestinal Adverse Reactions: Use has been associated with gastrointestinal adverse reactions, sometimes severe. WEGOVY is not recommended in patients with severe gastroparesis. (5.6)
•
Hypersensitivity Reactions: Anaphylactic reactions and angioedema have been reported postmarketing. Discontinue WEGOVY if suspected and promptly seek medical advice. (5.7)
•
Diabetic Retinopathy Complications in Patients with Type 2 Diabetes: Has been reported in trials with semaglutide. Patients with a history of diabetic retinopathy should be monitored. (5.8)
•
Heart Rate Increase: Monitor heart rate at regular intervals. (5.9)
•
Suicidal Behavior and Ideation: Monitor for depression or suicidal thoughts. Discontinue WEGOVY if symptoms develop. (5.10)
•
Pulmonary Aspiration During General Anesthesia or Deep Sedation: Has been reported in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures. Instruct patients to inform healthcare providers of any planned surgeries or procedures. (5.11)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are described below or elsewhere in the prescribing information:
•
Risk of Thyroid C-Cell Tumors [see Warnings and Precautions (5.1)]
•
Acute Pancreatitis [see Warnings and Precautions (5.2)]
•
Acute Gallbladder Disease [see Warnings and Precautions (5.3)]
•
Hypoglycemia [see Warnings and Precautions (5.4)]
•
Acute Kidney Injury due to Volume Depletion [see Warnings and Precautions (5.5)]
•
Severe Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.6)]
•
Hypersensitivity Reactions [see Warnings and Precautions (5.7)]
•
Diabetic Retinopathy Complications in Patients with Type 2 Diabetes [see Warnings and Precautions (5.8)]
•
Heart Rate Increase [see Warnings and Precautions (5.9)]
•
Suicidal Behavior and Ideation [see Warnings and Precautions (5.10)]
•
Pulmonary Aspiration During General Anesthesia or Deep Sedation [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Clinical Trials in Adults with Obesity or Overweight for Weight Reduction
WEGOVY 2.4 mg Subcutaneous Weekly Dosage
WEGOVY was evaluated for safety in 3 randomized, double-blind, placebo- controlled trials that included 2,116 adult patients with obesity or overweight treated with 2.4 mg WEGOVY for up to 68 weeks and a 7-week off-drug follow-up period [see Clinical Studies (14.2)]. Baseline characteristics included a mean age of 48 years, 71% female, 72% White, 14% Asian, 9% Black or African American, and 5% reported as other or unknown; and 85% were not Hispanic or Latino ethnicity, 13% were Hispanic or Latino ethnicity, and 2% reported as unknown. The baseline characteristics were 42% with hypertension, 19% with type 2 diabetes, 43% with dyslipidemia, 28% with a BMI greater than 40 kg/m2, and 4% with CV disease.
In these clinical trials, 6.8% of patients treated with 2.4 mg WEGOVY and 3.2% of patients treated with placebo permanently discontinued treatment as a result of adverse reactions. The most common adverse reactions leading to discontinuation were nausea (1.8% versus 0.2%), vomiting (1.2% versus 0%), and diarrhea (0.7% versus 0.1%) for WEGOVY and placebo, respectively.
Adverse reactions reported in clinical trials in adults and greater than or equal to 2% of WEGOVY-treated patients and more frequently than in placebo- treated patients are shown inTable 2.
**Table 2. Adverse Reactions (≥2% and Greater Than Placebo) in WEGOVY-treated Adults with Obesity or Overweight for Weight Reduction**
|
Placebo |
WEGOVY 2.4 mg | |
|---|---|---|
|
Nausea |
16 |
44 |
|
Diarrhea |
16 |
30 |
|
Vomiting |
6 |
24 |
|
Constipation |
11 |
24 |
|
Abdominal Paina |
10 |
20 |
|
Headache |
10 |
14 |
|
Fatigueb |
5 |
11 |
|
Dyspepsia |
3 |
9 |
|
Dizziness |
4 |
8 |
|
Abdominal Distension |
5 |
7 |
|
Eructation |
<1 |
7 |
|
Hypoglycemia in T2DMc |
2 |
6 |
|
Flatulence |
4 |
6 |
|
Gastroenteritis |
4 |
6 |
|
Gastroesophageal Reflux Disease |
3 |
5 |
|
Gastritisd |
1 |
4 |
|
Gastroenteritis Viral |
3 |
4 |
|
Hair Loss |
1 |
3 |
|
Dysesthesiae |
1 |
2 |
a Includes abdominal pain, abdominal pain upper, abdominal pain lower, gastrointestinal pain, abdominal tenderness, abdominal discomfort and epigastric discomfort
b Includes fatigue and asthenia
c Defined as blood glucose <54 mg/dL with or without symptoms of hypoglycemia or severe hypoglycemia (requiring the assistance of another person) in patients with type 2 diabetes not on concomitant insulin (Study 3, WEGOVY N=403, Placebo N=402). See text below for further information regarding hypoglycemia in patients with and without type 2 diabetes. T2DM = type 2 diabetes mellitus
d Includes chronic gastritis, gastritis, gastritis erosive, and reflux gastritis
e Includes paresthesia, hyperesthesia, burning sensation, allodynia, dysesthesia, skin burning sensation, pain of skin, and sensitive skin
In a CV outcomes trial, 8,803 patients were exposed to WEGOVY for a median of 37.3 months and 8,801 patients were exposed to placebo for a median of 38.6 months [see Clinical Studies (14.1)]. Safety data collection was limited to serious adverse events (including death), adverse events leading to discontinuation, and adverse events of special interest. Sixteen percent (16%) of WEGOVY-treated patients and 8% of placebo-treated patients, respectively, discontinued study drug due to an adverse event. Additional information from this trial is included in subsequent sections below when relevant.
Adverse Reactions in a Clinical Trial of Pediatric Patients Aged 12 Years and Older with Obesity for Weight Reduction
WEGOVY was evaluated in a 68-week, double-blind, randomized, parallel group, placebo-controlled, multi-center trial in 201 pediatric patients aged 12 years and older with obesity [see Clinical Studies (14.3)]. Baseline characteristics included a mean age of 15.4 years; 38% of patients were male; 79% were White, 8% were Black or African American, 2% were Asian, and 11% were of other or unknown race; and 11% were of Hispanic or Latino ethnicity. The mean baseline body weight was 107.5 kg, and mean BMI was 37 kg/m2.
Table 3 shows adverse reactions reported in greater than or equal to 3% of WEGOVY-treated pediatric patients and more frequently than in the placebo group from a study in pediatric patients aged 12 years and older.
**Table 3. Adverse Reactions (≥3% and Greater than Placebo) in WEGOVY-Treated Pediatric Patients Aged 12 Years and Older with Obesity for Weight Reduction**
|
Placebo |
WEGOVY 2.4 mg | |
|---|---|---|
|
Nausea |
18 |
42 |
|
Vomiting |
10 |
36 |
|
Diarrhea |
19 |
22 |
|
Headache |
16 |
17 |
|
Abdominal Pain |
6 |
15 |
|
Nasopharyngitis |
10 |
12 |
|
Dizziness |
3 |
8 |
|
Gastroenteritis |
3 |
7 |
|
Constipation |
2 |
6 |
|
Gastroesophageal Reflux Disease |
2 |
4 |
|
Sinusitis |
2 |
4 |
|
Urinary tract infection |
2 |
4 |
|
Ligament sprain |
2 |
4 |
|
Anxiety |
2 |
4 |
|
Hair Loss |
0 |
4 |
|
Cholelithiasis |
0 |
4 |
|
Eructation |
0 |
4 |
|
Influenza |
0 |
3 |
|
Rash |
0 |
3 |
|
Urticaria |
0 |
3 |
Adverse Reactions in Clinical Trials in Adults with MASH
The safety of WEGOVY was evaluated in a randomized, double-blind, placebo- controlled trial (Study 8) that included 1,195 adult patients with MASH, including 800 patients who were exposed to WEGOVY for a median of 95.3 weeks and 395 patients who were exposed to placebo for a median of 83.1 weeks [see Clinical Studies (14.4)].
The most commonly reported adverse reactions were consistent with the other approved WEGOVY indications (seeTable 2). There is limited information in patients with MASH and a BMI <25 kg/m2. Additional information from the MASH trial is included in subsequent sections when notable. Unless indicated, the incidence of the adverse reactions in MASH patients was similar to other approved indications.
Other Adverse Reactions in Adults and/or Pediatric Patients
Acute Pancreatitis
In WEGOVY clinical trials in adults for weight reduction, acute pancreatitis was confirmed by adjudication in 4 WEGOVY-treated patients (0.2 cases per 100 patient years) and 1 in placebo-treated patients (less than 0.1 cases per 100 patient years). One additional case of acute pancreatitis was confirmed in a patient treated with WEGOVY in another clinical trial.
Acute Gallbladder Disease
In WEGOVY clinical trials in adults for weight reduction, cholelithiasis was reported by 1.6% of WEGOVY-treated patients and 0.7% of placebo-treated patients. Cholecystitis was reported by 0.6% of WEGOVY-treated adult patients and 0.2% of placebo-treated patients. In a clinical trial in pediatric patients aged 12 years and older for weight reduction [see Clinical Studies (14.3)], cholelithiasis was reported by 3.8% of WEGOVY-treated patients and 0% placebo-treated patients. Cholecystitis was reported by 0.8% of WEGOVY-treated pediatric patients and 0% placebo-treated patients.
Hypoglycemia
Patients with Type 2 Diabetes
In a trial of adult patients with type 2 diabetes and BMI greater than or equal to 27 kg/m2 for weight reduction, clinically significant hypoglycemia (defined as a plasma glucose less than 54 mg/dL) was reported in 6.2% of WEGOVY-treated patients versus 2.5% of placebo-treated patients. A higher rate of clinically significant hypoglycemic episodes was reported with WEGOVY (semaglutide 2.4 mg) versus semaglutide 1 mg (10.7 vs. 7.2 episodes per 100 patient years of exposure, respectively); the rate in the placebo-treated group was 3.2 episodes per 100 patient years of exposure. In addition, one episode of severe hypoglycemia requiring intravenous glucose was reported in a WEGOVY-treated patient versus none in placebo-treated patients. The risk of hypoglycemia was increased when WEGOVY was used with a sulfonylurea.
Patients without Type 2 Diabetes
Episodes of hypoglycemia have been reported with GLP-1 receptor agonists in adult patients without type 2 diabetes mellitus. In WEGOVY clinical trials in adult patients without type 2 diabetes mellitus for weight reduction, there was no systematic capturing or reporting of hypoglycemia.
In a CV outcomes trial in adult patients without type 2 diabetes, 3 episodes of serious hypoglycemia were reported in WEGOVY-treated patients versus 1 episode in placebo. Patients with a history of bariatric surgery (a risk factor for hypoglycemia) had more events of serious hypoglycemia while taking WEGOVY (2.3%, 2/87) than placebo (0%, 0/97).
Acute Kidney Injury
Acute kidney injury occurred in clinical trials for weight reduction in 7 adult patients (0.4 cases per 100 patient years) receiving WEGOVY versus 4 patients (0.2 cases per 100 patient years of exposure) receiving placebo. Some of these adverse reactions occurred in association with gastrointestinal adverse reactions or dehydration. In addition, 2 patients treated with WEGOVY had acute kidney injury with dehydration in other clinical trials. The risk of renal adverse reactions with WEGOVY was increased in adult patients with a history of renal impairment (trials included 65 patients with a history of moderate or severe renal impairment at baseline), and occurred more frequently during dose titration.
Retinal Disorders in Patients with Type 2 Diabetes
In a trial of adult patients with type 2 diabetes and BMI greater than or equal to 27 kg/m2 for weight reduction, retinal disorders were reported by 6.9% of patients treated with WEGOVY (semaglutide 2.4 mg), 6.2% of patients treated with semaglutide 1 mg, and 4.2% of patients treated with placebo. The majority of events were reported as diabetic retinopathy (4%, 2.7%, and 2.7%, respectively) and non-proliferative retinopathy (0.7%, 0%, and 0%, respectively).
Increase in Heart Rate
Mean increases in resting heart rate of 1 to 4 beats per minute (bpm) were observed with routine clinical monitoring in WEGOVY-treated adult patients compared to placebo in clinical trials for weight reduction. In weight reduction trials in which adult patients were randomized prior to dose- escalation, more patients treated with WEGOVY, compared with placebo, had maximum changes from baseline at any visit of 10 to 19 bpm (41% versus 34%, respectively) and 20 bpm or more (26% versus 16%, respectively). In a clinical trial for weight reduction in pediatric patients aged 12 years and older with normal baseline heart rate, more patients treated with WEGOVY compared to placebo had maximum changes in heart rate of 20 bpm or more (54% versus 39%).
Hypotension and Syncope
Adverse reactions related to hypotension (hypotension, orthostatic hypotension, and decreased blood pressure) were reported in 1.3% of WEGOVY- treated adult patients versus 0.4% of placebo-treated patients and syncope was reported in 0.8% of WEGOVY-treated patients versus 0.2% of placebo-treated patients in clinical trials for weight reduction. Some reactions were related to gastrointestinal adverse reactions and volume loss associated with WEGOVY. Hypotension and orthostatic hypotension were more frequently seen in patients on concomitant antihypertensive therapy. In a clinical trial in pediatric patients aged 12 years and older for weight reduction, hypotension was reported in 2.3% of WEGOVY-treated patients versus 0% in placebo-treated patients.
Appendicitis
Appendicitis (including perforated appendicitis) occurred in 10 (0.5%) WEGOVY- treated adult patients and 2 (0.2%) patients receiving placebo in clinical trials for weight reduction.
Gastrointestinal Adverse Reactions
In clinical trials in adults for weight reduction, 73% of WEGOVY-treated patients and 47% of patients receiving placebo reported gastrointestinal adverse reactions, including severe reactions that were reported more frequently among patients receiving WEGOVY (4.1%) than placebo (0.9%). The most frequently reported reactions were nausea (44% vs. 16%), vomiting (25% vs. 6%), and diarrhea (30% vs. 16%). Other reactions that occurred at a higher incidence among WEGOVY-treated adult patients included dyspepsia, abdominal pain, abdominal distension, eructation, flatulence, gastroesophageal reflux disease, gastritis, hemorrhoids, and hiccups. These reactions were most frequently reported during dosage escalation.
In the pediatric clinical trial for weight reduction, 62% of WEGOVY-treated patients and 42% of placebo-treated patients reported gastrointestinal adverse reactions. The most frequently reported reactions were nausea (42% vs. 18%), vomiting (36% vs. 10%), and diarrhea (22% vs. 19%). Other gastrointestinal- related reactions that occurred at a higher incidence than placebo among WEGOVY-treated pediatric patients included abdominal pain, constipation, eructation, gastroesophageal reflux disease, dyspepsia, and flatulence.
Permanent discontinuation of treatment as a result of a gastrointestinal adverse reaction occurred in 4.3% of WEGOVY-treated adult patients versus 0.7% of placebo-treated patients. In a pediatric clinical trial for weight reduction, 2.3% of patients treated with WEGOVY versus 1.5% of patients who received placebo discontinued treatment as a result of gastrointestinal adverse reactions.
Injection Site Reactions
In clinical trials in adults for weight reduction, 1.4% of WEGOVY-treated patients and 1% of patients receiving placebo experienced injection site reactions (including injection site pruritus, erythema, inflammation, induration, and irritation).
Hypersensitivity Reactions
Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported with WEGOVY.
In a pediatric clinical trial for weight reduction, rash was reported in 3% of WEGOVY-treated patients and 0% of placebo-treated patients, and urticaria was reported in 3% of WEGOVY-treated patients and 0% of placebo-treated patients.
In adult clinical trials for weight reduction, allergic reactions occurred in 16% (8/50) of WEGOVY-treated patients with anti-semaglutide antibodies and in 7% (114/1,659) of WEGOVY-treated patients who did not develop anti-semaglutide antibodies [see Clinical Pharmacology (12.6)].
Fractures
In the CV outcomes trial in adults, more fractures of the hip and pelvis were reported on WEGOVY than on placebo in female patients: 1% (24/2,448) vs. 0.2% (5/2,424), and in patients ages 75 years and older: 2.4% (17/703) vs. 0.6% (4/663), respectively.
In a clinical trial in adults with MASH, fractures occurred in 4.4% of WEGOVY- treated patients (2.6 cases per 100 patient years) compared to 3.3% of placebo-treated patients (2 cases per 100 patient years). Fractures were reported in both males and females with a median age of 61 years (range, 44-75).
Urolithiasis
In a CV outcomes trial, 1.2% of WEGOVY-treated patients and 0.8% of patients receiving placebo reported urolithiasis, including serious reactions that were reported more frequently among patients receiving WEGOVY (0.6%) than placebo (0.4%).
Dysgeusia
In clinical trials in adults for weight reduction, 1.7% of WEGOVY-treated patients and 0.5% of placebo-treated patients reported dysgeusia.
Laboratory Abnormalities
Amylase and Lipase
Adult and pediatric patients treated with WEGOVY had a mean increase from baseline in amylase of 15 to 16% and lipase of 39% in clinical trials for weight reduction. These changes were not observed in the placebo group.
In a clinical trial in adults with MASH, increases in lipase greater than 3 times the upper limit of normal (ULN) occurred in 4.7% (35/750) of WEGOVY- treated patients compared with 1.3% (5/374) of placebo-treated patients. The clinical significance of elevations in lipase or amylase with WEGOVY is unknown in the absence of other signs and symptoms of pancreatitis.
Liver Tests
In a pediatric clinical trial for weight reduction, increases in alanine aminotransferase (ALT) greater than or equal to 5 times the ULN were observed in 4 (3%) WEGOVY-treated patients compared with 0% of placebo-treated patients. In some patients, increases in ALT and AST were associated with other confounding factors (such as gallstones). In the CV outcomes trial in adults, increases in total bilirubin greater than or equal to 3 times the ULN were observed in 0.3% (30/8,585) of WEGOVY-treated patients versus 0.2% (14/8,579) of placebo-treated patients.
6.2 Postmarketing Experience
The following adverse reactions have been reported during post-approval use of semaglutide, the active ingredient of WEGOVY. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: acute pancreatitis and necrotizing pancreatitis, sometimes resulting in death; ileus
Hypersensitivity: anaphylaxis, angioedema, rash, urticaria
Pulmonary: Pulmonary aspiration has occurred in patients receiving GLP-1 receptor agonists undergoing
elective surgeries or procedures requiring general anesthesia or deep sedation
Renal and Urinary Disorders: acute kidney injury
Most common adverse reactions (incidence ≥5%) in adults or pediatric patients aged 12 years and older are: nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, dyspepsia, dizziness, abdominal distension, eructation, hypoglycemia in patients with type 2 diabetes, flatulence, gastroenteritis, gastroesophageal reflux disease, and nasopharyngitis. (6.1)
**To report SUSPECTED ADVERSE REACTIONS, contactNovo Nordisk Inc., at 1-833-934-6891 or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Concomitant Use with Insulin or an Insulin Secretagogue (e.g.,
Sulfonylurea)
WEGOVY lowers blood glucose and can cause hypoglycemia. The risk of hypoglycemia is increased when WEGOVY is used in combination with insulin or insulin secretagogues (e.g., sulfonylureas). The addition of WEGOVY in patients treated with insulin has not been evaluated.
When initiating WEGOVY, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia [see Warnings and Precautions (5.4), Adverse Reactions (6.1)].
7.2 Oral Medications
WEGOVY causes a delay of gastric emptying and thereby has the potential to impact the absorption of concomitantly administered oral medications. In clinical pharmacology trials with semaglutide 1 mg, semaglutide did not affect the absorption of orally administered medications [see Clinical Pharmacology (12.3)]. Nonetheless, monitor the effects of oral medications concomitantly administered with WEGOVY.
WEGOVY delays gastric emptying. May impact absorption of concomitantly administered oral medications. Use with caution. (7.2)
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Cardiovascular Outcomes Trial in Adult Patients with Cardiovascular
Disease and Either Obesity or Overweight
Overview of Clinical Trial
Study 1 (NCT03574597) was a multi-national, multi-center, placebo-controlled,
double-blind trial to determine the effect of WEGOVY relative to placebo on
major adverse CV events (MACE) when added to current standard of care, which
included management of CV risk factors and individualized healthy lifestyle
counseling (including diet and physical activity). The primary endpoint, MACE,
was the time to first occurrence of a three-part composite outcome which
included CV death, non-fatal myocardial infarction, and non-fatal stroke.
All patients were 45 years or older, with an initial BMI of 27 kg/m2 or greater and established CV disease (prior myocardial infarction, prior stroke, or peripheral arterial disease). Patients with a history of type 1 or type 2 diabetes were excluded. Concomitant CV therapies could be adjusted, at the discretion of the investigator, to ensure participants were treated according to the current standard of care for patients with established CV disease.
In this trial, 17,604 patients were randomized to WEGOVY or placebo. At baseline, the mean age was 62 years (range 45 - 93), 72% were male, 84% were White, 4% were Black or African American, and 8% were Asian, and 10% were Hispanic or Latino. Mean baseline body weight was 97 kg and mean BMI was 33 kg/m2. At baseline, prior myocardial infarction was reported in 76% of randomized individuals, prior stroke in 23%, and peripheral arterial disease in 9%. Heart failure was reported in 24% of patients. At baseline, CV disease and risk factors were managed with lipid-lowering therapy (90%), platelet aggregation inhibitors (86%), angiotensin converting enzyme inhibitors or angiotensin II receptor blockers (74%), and beta blockers (70%). A total of 10% had moderate renal impairment (eGFR 30 to <60 mL/min/1.73m2) and 0.4% had severe renal impairment eGFR <30 mL/min/1.73m2.
Results
In total, 96.9% of patients completed the trial, and vital status was available for 99.4% of patients. The median follow-up duration was 41.8 months. A total of 31% of WEGOVY-treated patients and 27% of placebo-treated patients permanently discontinued study drug.
For the primary analysis, a Cox proportional hazards model was used to test for superiority. Type 1 error was controlled across multiple tests.
WEGOVY significantly reduced the risk for first occurrence of MACE. The estimated hazard ratio (95% CI) was 0.8 (0.72, 0.9) (seeFigure 4 and Table 4).
**Figure 4.**Cumulative Incidence Function: Time to First Occurrence of MACE in Study 1
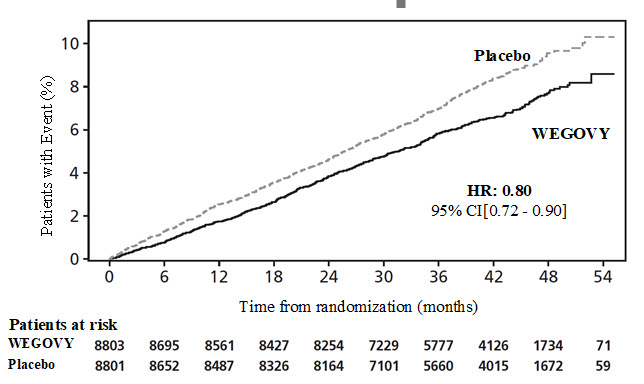
Data from the in-trial period. Cumulative incidence estimates are based on time from randomization to first EAC-confirmed CV death, non-fatal myocardial infarction, or non-fatal stroke with non-CV death modeled as competing risk using the Aalen-Johansen estimator. Patients without events of interest were censored at the end of their in-trial observation period. Time from randomization to first CV death, non-fatal myocardial infarction, or non-fatal stroke was analyzed using a Cox proportional hazards model with treatment as categorical fixed factor. The hazard ratio and confidence interval are adjusted for the group sequential design using the likelihood ratio ordering.
HR: Hazard ratio; CI: confidence interval; CV: cardiovascular
The treatment effect for the primary composite endpoint, its components, and other relevant endpoints in Study 1 are shown inTable 4.
Table 4. Treatment Effect for MACE and Other Events in Study 1
|
Patients with events n (%) | |||
|
Placebo N=8,801 |
WEGOVY N=8,803 |
Hazard Ratio (95% CI) | |
|
Primary composite endpoint | |||
|
Composite of CV death, non-fatal myocardial infarction, or non-fatal stroke1 |
701 (8%) |
569 (6.5%) |
0.8 (0.72; 0.9)*2 |
|
Key secondary endpoints | |||
|
CV death3 |
262 (3%) |
223 (2.5%) |
0.85 (0.71; 1.01) |
|
All-cause death4 |
458 (5.2%) |
375 (4.3%) |
0.81 (0.71; 0.93) |
|
Other secondary endpoints | |||
|
Fatal or non-fatal myocardial infarction5 |
334 (3.8%) |
243 (2.8%) |
0.72 (0.61; 0.85) |
|
Fatal or non-fatal stroke5 |
178 (2%) |
160 (1.8%) |
0.89 (0.72; 1.11) |
*p-value <0.001, one-sided p-value
1Primary endpoint
2Adjusted for group sequential design using the likelihood ratio ordering.
3CV death was the first confirmatory secondary endpoint in the testing hierarchy and superiority was not confirmed.
4Confirmatory secondary endpoint. Not statistically significant based on the prespecified testing hierarchy.
5Not included in the prespecified testing hierarchy for controlling type-I error.
NOTE: Time to first event was analyzed in a Cox proportional hazards model with treatment as factor. For patients with multiple events, only the first event contributed to the composite endpoint.
Table 5. Mean Changes in Anthropometry and Cardiometabolic Parameters at Week 104 in Study 1****1,2
|
PLACEBO |
WEGOVY | ||||
|
Baseline |
Change from Baseline (LSMean) |
Baseline |
Change from Baseline (LSMean) |
Difference from Placebo (LSMean) | |
|
Body Weight (kg) |
96.8 |
-0.93 |
96.5 |
-9.43 |
-8.53 |
|
Waist Circumference (cm) |
111.4 |
-1 |
111.3 |
-7.6 |
-6.5 |
|
Systolic Blood Pressure (mmHg) |
131 |
-0.5 |
131 |
-3.8 |
-3.3 |
|
Diastolic Blood Pressure (mmHg) |
79 |
-0.5 |
79 |
-1 |
-0.5 |
|
Heart Rate |
69 |
0.7 |
69 |
3.8 |
3.1 |
|
HbA1c (%) |
5.8 |
0 |
5.8 |
-0.3 |
-0.3 |
|
Total Cholesterol (mg/dL)4 |
156 |
-1.9 |
155.5 |
-4.6 |
-2.8 |
|
LDL Cholesterol (mg/dL)4 |
78.5 |
-3.1 |
78.5 |
-5.3 |
-2.2 |
|
HDL Cholesterol (mg/dL)4 |
44.2 |
0.6 |
44.1 |
4.9 |
4.2 |
|
Triglycerides (mg/dL)4 |
139.5 |
-3.2 |
138.6 |
-18.3 |
-15.6 |
1Parameters listed in the table were not included in the pre-specified hierarchical testing.
2Responses were analyzed using an ANCOVA with treatment as fixed factor and baseline value as covariate. Before analysis, missing data were multiple imputed. The imputation model (linear regression) was done separately for each treatment arm and included baseline value as a covariate and was fitted to all subjects with a measurement regardless of treatment status at week 104.
3For body weight the ‘change from baseline’ and ‘difference to placebo’ the unit is percentage change from baseline.
4Baseline value is the geometric mean.
The reduction of MACE with WEGOVY was not impacted by age, sex, race, ethnicity, BMI at baseline, or level of renal function impairment.
14.2 Weight Reduction and Long-term Maintenance Studies in Adults with
Obesity or Overweight
Overview of Clinical Studies in Adults
The safety and efficacy of WEGOVY for weight reduction and long-term
maintenance of body weight in conjunction with a reduced calorie diet and
increased physical activity were studied in three 68-week, randomized, double-
blind, placebo-controlled trials; one 68-week, randomized, double-blind,
placebo withdrawal trial; and one 68-week, randomized, double-blind trial that
investigated 2 different doses of WEGOVY versus placebo. In Studies 2
(NCT#03548935), 3 (NCT#03552757), and 4 (NCT#03611582), WEGOVY or matching
placebo was escalated to 2.4 mg subcutaneous weekly during a 16-week period
followed by 52 weeks on maintenance dose. In Study 5 (NCT#03548987), WEGOVY
was escalated during a 20-week run-in period, and patients who reached a
WEGOVY 2.4 mg subcutaneous weekly dosage after the run-in period were
randomized to either continued treatment with WEGOVY or placebo for 48 weeks.
In Study 6 (NCT#03811574), WEGOVY was escalated to 1.7 mg or 2.4 mg
subcutaneous weekly dosages or placebo over 12 to 16 weeks followed by 52
weeks on either maintenance dose.
In Studies 2, 3, and 5, all patients received instruction for a reduced calorie diet (approximately 500 kcal/day deficit) and increased physical activity counseling (recommended to a minimum of 150 min/week) that began with the first dose of study medication or placebo and continued throughout the trial. In Study 4, patients received an initial 8-week low-calorie diet (total energy intake 1,000 to 1,200 kcal/day) followed by 60 weeks of a reduced calorie diet (1,200-1,800 kcal/day) and increased physical activity (100 mins/week with gradual increase to 200 mins/week).
Study 2 was a 68-week trial that enrolled 1,961 patients with obesity (BMI greater than or equal to 30 kg/m2) or with overweight (BMI 27 to 29.9 kg/m2) and at least one weight-related comorbid condition, such as treated or untreated dyslipidemia or hypertension; patients with type 2 diabetes mellitus were excluded. Patients were randomized in a 2:1 ratio to either WEGOVY or placebo. At baseline, mean age was 46 years (range 18 to 86), 74% were female, 75% were White, 13% were Asian and 6% were Black or African American. A total of 12% were Hispanic or Latino ethnicity. Mean baseline body weight was 105.3 kg and mean BMI was 37.9 kg/m2.
Study 3 was a 68-week trial that enrolled 807 patients with type 2 diabetes and BMI greater than or equal to 27 kg/m2. Patients included in the trial had HbA1c 7-10% and were treated with either: diet and exercise alone or 1 to 3 oral anti‑diabetic drugs (metformin, sulfonylurea, glitazone or sodium-glucose co-transporter 2 inhibitor). Patients were randomized in a 1:1 ratio to receive either WEGOVY or placebo. At baseline, the mean age was 55 years (range 19 to 84), 51% were female, 62% were White, 26% were Asian and 8% were Black or African American. A total of 13% were Hispanic or Latino ethnicity. Mean baseline body weight was 99.8 kg and mean BMI was 35.7 kg/m2.
Study 4 was a 68-week trial that enrolled 611 patients with obesity (BMI greater than or equal to 30 kg/m2) or with overweight (BMI 27 to 29.9 kg/m2) and at least one weight-related comorbid condition such as treated or untreated dyslipidemia or hypertension; patients with type 2 diabetes mellitus were excluded. The patients were randomized in a 2:1 ratio to receive either WEGOVY or placebo. At baseline, the mean age was 46 years, 81% were female, 76% were White, 19% were Black or African American and 2% were Asian. A total of 20% were Hispanic or Latino ethnicity. Mean baseline body weight was 105.8 kg and mean BMI was 38 kg/m2.
Study 5 was a 68-week trial that enrolled 902 patients with obesity (BMI greater than or equal to 30 kg/m2) or with overweight (BMI 27 to 29.9 kg/m2) and at least one weight-related comorbid condition such as treated or untreated dyslipidemia or hypertension; patients with type 2 diabetes mellitus were excluded. Mean body weight at baseline for the 902 patients was 106.8 kg and mean BMI was 38.3 kg/m². All patients received WEGOVY during the run-in period of 20 weeks that included 16 weeks of dose escalation. Trial product was permanently discontinued before randomization in 99 of 902 patients (11%); the most common reason was adverse reactions (n=48, 5.3%); 803 patients reached WEGOVY 2.4 mg and were then randomized in a 2:1 ratio to either continue on WEGOVY or receive placebo. Among the 803 randomized patients, the mean age was 46 years, 79% were female, 84% were White, 13% were Black or African American, and 2% Asian. A total of 8% were Hispanic or Latino ethnicity. Mean body weight at randomization (week 20) was 96.1 kg and mean BMI at randomization (week 20) was 34.4 kg/m2.
Study 6 was a 68-week trial that enrolled 401 East-Asian patients (Japan and South Korea) with BMI greater than or equal to 35 kg/m2 and at least one weight-related comorbid condition or with BMI 27 to 34.9 kg/m2 and at least two weight-related comorbid conditions. The patients were randomized 2:1:1 to receive WEGOVY 2.4 mg, WEGOVY 1.7 mg, or placebo. At baseline, the mean age was 51 years, 63% were male, and all patients were Asian. Mean baseline body weight was 87.5 kg and mean BMI was 31.9 kg/m2. At baseline, 24.7% of patients had type 2 diabetes mellitus.
Results
The proportions of patients who discontinued study drug in Studies 2, 3, and 4
was 16% for the WEGOVY-treated group and 19.1% for the placebo-treated group,
and 6.8% of patients treated with WEGOVY and 3.2% of patients treated with
placebo discontinued treatment due to an adverse reaction [see Adverse Reactions (6.1)]. In Study 5, the proportions of patients who discontinued
study drug were 5.8% and 11.6% for WEGOVY and placebo, respectively. In Study
6, the proportions of patients who discontinued study drug were 7.9%, 6.5%,
and 3% for WEGOVY 1.7 mg, WEGOVY 2.4 mg, and placebo, respectively.
For Studies 2, 3, and 4, the primary efficacy parameters were mean percent change in body weight and the percentages of patients achieving greater than or equal to 5% weight loss from baseline to week 68.
After 68 weeks, treatment with WEGOVY resulted in a statistically significant reduction in body weight compared with placebo. Greater proportions of patients treated with WEGOVY achieved 5%, 10% and 15% weight loss than those treated with placebo as shown inTable 6.
**Table 6. Changes in Body Weight at Week 68 in Studies 2, 3, and 4**
|
Study 2 (Obesity or overweight with comorbidity) |
Study 3 (Type 2 diabetes with obesity or overweight) |
Study 4 (Obesity or overweight with comorbidity undergoing intensive lifestyle therapy) | ||||
|---|---|---|---|---|---|---|
|
Intention-to-Treat1 |
PLACEBO |
WEGOVY |
PLACEBO |
WEGOVY |
PLACEBO |
WEGOVY |
|
Body Weight | ||||||
|
105.2 |
105.4 |
100.5 |
99.9 |
103.7 |
106.9 |
|
-2.4 |
-14.9 |
-3.4 |
-9.6 |
-5.7 |
-16 |
|
-12.4 |
-6.2 |
-10.3 | |||
|
% of Patients losing greater than or equal to 5% body weight |
31.1 |
83.5 |
30.2 |
67.4 |
47.8 |
84.8 |
|
52.4 (48.1; 56.7)* |
37.2 (30.7; 43.8)* |
37 (28.9; 45.2)* | |||
|
% of Patients losing greater than or equal to 10% body weight |
12 |
66.1 |
10.2 |
44.5 |
27.1 |
73 |
|
54.1 (50.4; 57.9)* |
34.3 (28.4; 40.2)* |
45.9 (38; 53.7)* | |||
|
% of Patients losing greater than or equal to 15% body weight |
4.8 |
47.9 |
4.3 |
25.1 |
13.2 |
53.4 |
|
43.1 (39.8; 46.3)* |
20.7 (15.7; 25.8)* |
40.2 (33.1; 47.3)* |
LSMean = least squares mean; CI = confidence interval
1The intent-to-treat population includes all randomized patients. In Study 2, at week 68, the body weight was missing for 7.2% and 11.9% of patients randomized to WEGOVY and placebo, respectively. In Study 3, at week 68, the body weight was missing for 4% and 6.7% of patients randomized to WEGOVY and placebo, respectively. In Study 4, at week 68, the body weight was missing for 8.4% and 7.4% of patients randomized to WEGOVY and placebo, respectively. Missing data were imputed from retrieved subjects of the same randomized treatment arm (RD-MI).
- p<0.0001 (unadjusted 2-sided) for superiority.
For Study 5, the primary efficacy parameter was mean percent change in body weight from randomization (week 20) to week 68.
From randomization (week 20) to week 68, treatment with WEGOVY resulted in a statistically significant reduction in body weight compared with placebo (Table 7). Because patients who discontinued WEGOVY during titration and those who did not reach the 2.4 mg weekly dose were not eligible for the randomized treatment period, the results may not reflect the experience of patients in the general population who are first starting WEGOVY.
**Table 7. Changes in Body Weight at Week 68 in Study 5 (Obesity or Overweight with Comorbidity after 20-week Run-in)**
|
WEGOVY N=803****1 | ||
|
Body Weight (only randomized patients) | ||
|
107.2 | |
|
PLACEBO N=268 |
WEGOVY N=535 | |
|
Body Weight | ||
|
95.4 (22.7) |
96.5 (22.5) |
|
6.9 |
-7.9 |
|
-14.8 (-16; -13.5)* |
LSMean = least squares mean; CI = confidence interval
1902 patients were enrolled at week 0 with a mean baseline body weight of 106.8 kg. The intent-to-treat population includes all randomized patients. At week 68, the body weight was missing for 2.8% and 6.7% of patients randomized to WEGOVY and placebo, respectively. Missing data were imputed from retrieved subjects of the same randomized treatment arm (RD-MI).
*p<0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
For Study 6, the primary efficacy parameters were mean percent change in body weight and the percentage of patients achieving greater than or equal to 5% weight loss from baseline to week 68.
After 68 weeks, treatment with WEGOVY 1.7 mg and 2.4 mg resulted in a statistically significant reduction in body weight compared with placebo. Greater proportions of patients treated with WEGOVY achieved 5%, 10%, and 15% weight loss than those treated with placebo as shown inTable 8.
Table 8. Changes in Body Weight at Week 68 in Study 6 in East-Asian Patients (WEGOVY 1.7 mg)
|
Study 6 (BMI ≥35 kg/m2with at least one comorbidity or BMI 27-34.9 kg/m2with at least two comorbidities) | |||
|
Intention-to-treat1 |
PLACEBO N=101 |
WEGOVY 1.7 mg N=101 |
WEGOVY 2.4 mg N=199 |
|
Body Weight | |||
|
90.2 |
86.1 |
86.9 |
|
-2.1 |
-9.6 |
-13.2 |
|
-7.5 (-9.6; -5.4)* |
-11.1 (-12.9; -9.2)* | |
|
% of Patients losing greater than or equal to 5% body weight |
19.4 |
72.8 |
84 |
|
53.3 (41; 65.6)* |
64.5 (54.8; 74.3)* | |
|
% of Patients losing greater than or equal to 10% body weight |
4.5 |
39.1 |
59.9 |
|
34.5 (23.9; 45.1)* |
55.4 (47.3; 63.6)* | |
|
% of Patients losing greater than or equal to 15% body weight |
2.6 |
20.8 |
38.2 |
|
18.2 (9.8; 26.7)* |
35.6 (27.9; 43.3)* |
LSMean = least squares mean; CI = confidence interval
1The intent-to-treat population includes all randomized patients. At baseline,
24.7% of patients had type 2 diabetes mellitus. At week 68, the body weight
was missing for 3%, 3%, and 1% of patients randomized to WEGOVY 1.7 mg, WEGOVY
2.4 mg, and placebo, respectively. Missing data were imputed from retrieved
subjects of the same randomized treatment arm (RD-MI).
*p<0.0001 (unadjusted 2-sided) for superiority.
A reduction in body weight was observed with WEGOVY irrespective of age, sex, race, ethnicity, BMI at baseline, body weight (kg) at baseline, and level of renal function impairment.
The cumulative frequency distributions of change in body weight are shown in Figure 5 andFigure 6 for Studies 2 and 3. One way to interpret this figure is to select a change in body weight of interest on the horizontal axis and note the corresponding proportions of patients (vertical axis) in each treatment group who achieved at least that degree of weight loss. For example, note that the vertical line arising from ‑10% in Study 2 intersects the WEGOVY and placebo curves at approximately 66%, and 12%, respectively, which correspond to the values shown inTable 6.
**Figure 5.****Change in body weight (%) from baseline to week 68 (Study 2)**
****
****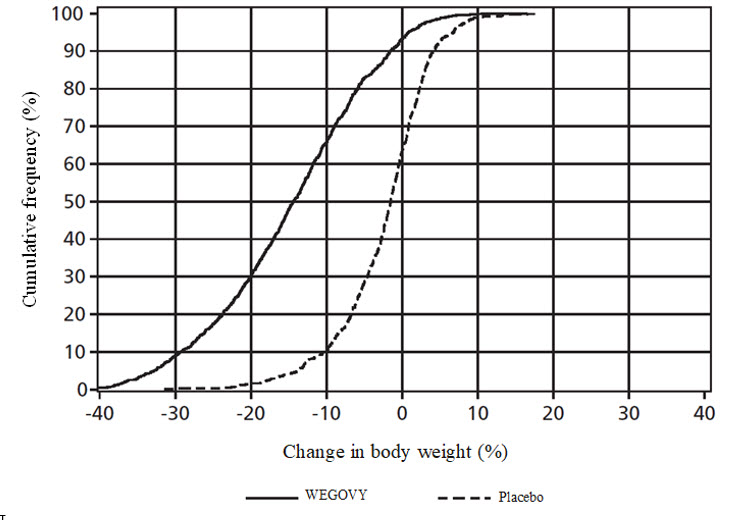
Observed data from in-trial period including imputed data for missing observations (RD-MI).
**Figure 6. Change in body weight (%) from baseline to week 68 (Study 3)**
****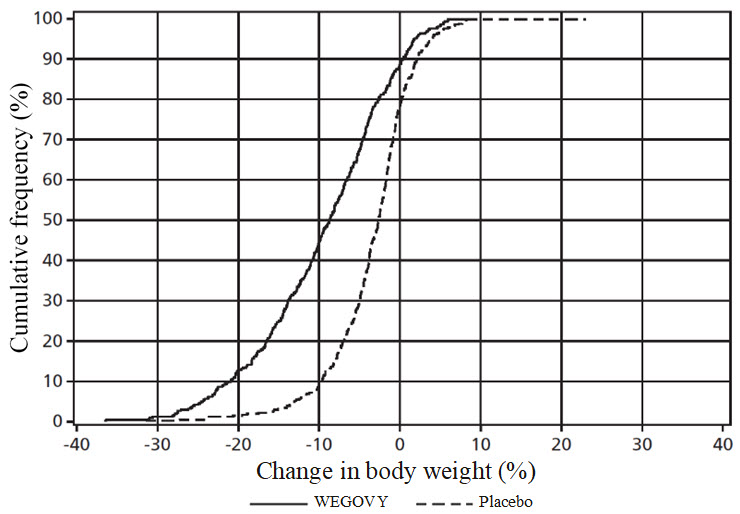
Observed data from in-trial period including imputed data for missing observations (RD-MI).
The time courses of weight loss with WEGOVY and placebo from baseline through week 68 are depicted inFigure 7,Figure 8 andFigure 9.
**Figure 7.****Change from baseline (%) in body weight (Study 2 on left and Study 3 on right)**
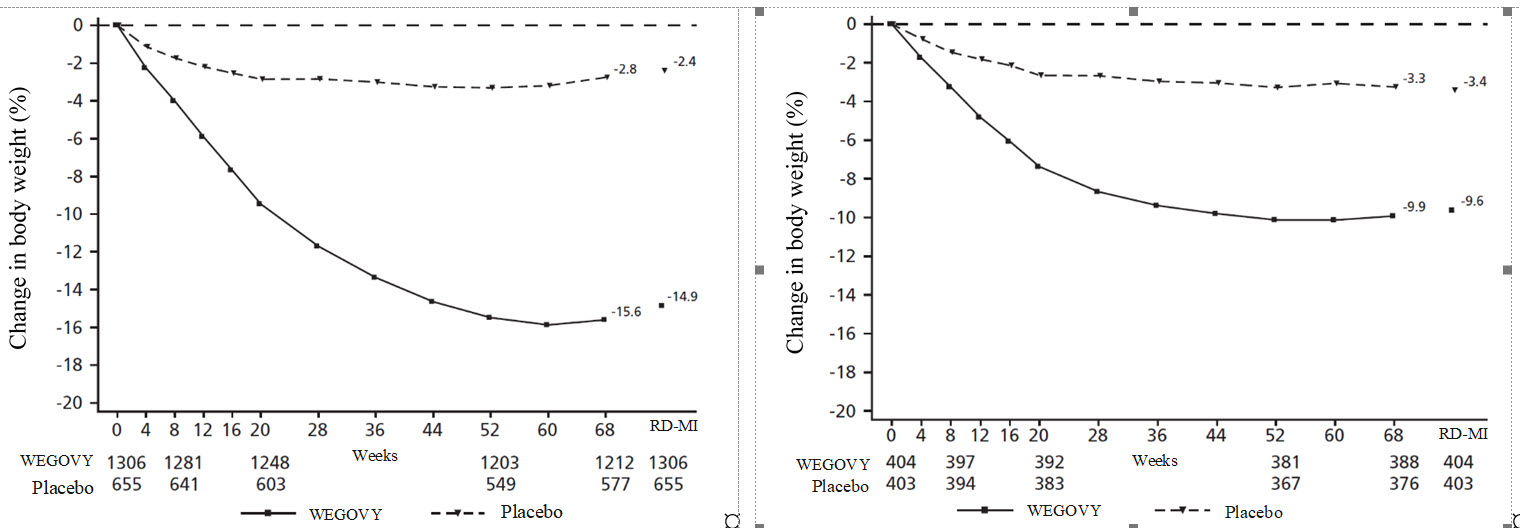
Observed values for patients completing each scheduled visit, and estimates with multiple imputations from retrieved dropouts (RD-MI)
**Figure 8. Change from baseline (%) in body weight (Study 4 on left and Study 5****a**** on right)**
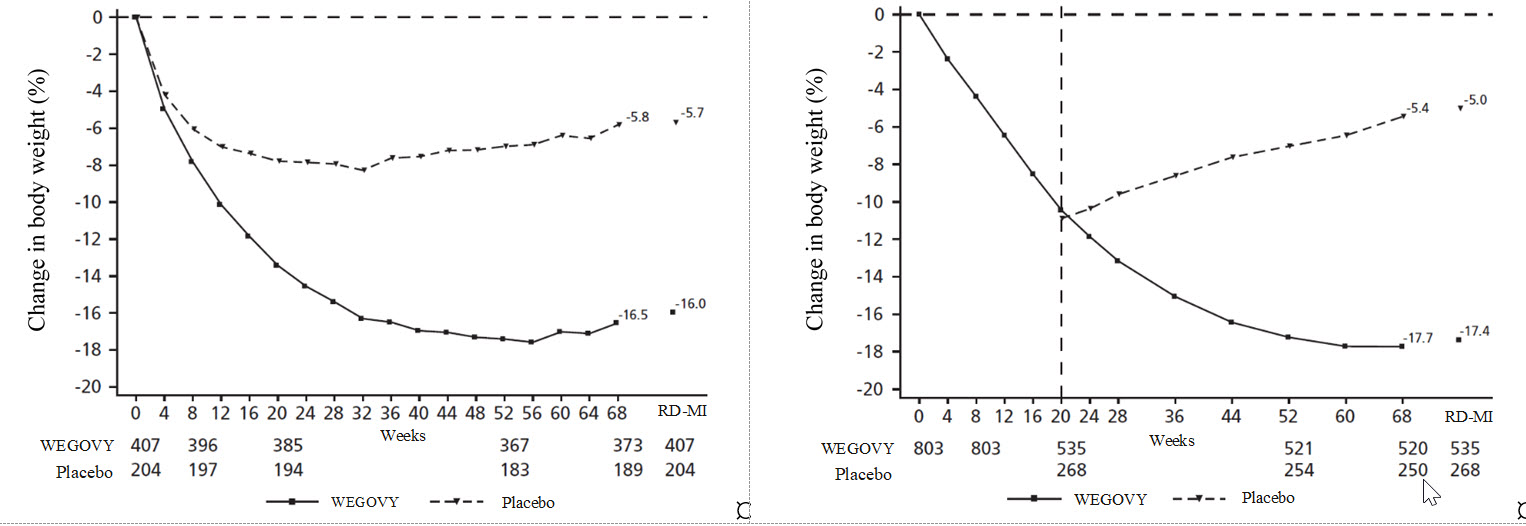
Observed values for patients completing each scheduled visit, and estimates
with multiple imputations from retrieved dropouts (RD-MI)
aChange from week 0 was not a primary endpoint in study 5. Dotted line
indicates time of randomization. Randomized patients (shown) do not include 99
patients that discontinued during the 20-week run-in period.
**Figure 9. Change in body weight (%) from baseline to week 68 (Study 6 in East-Asian Patients)**
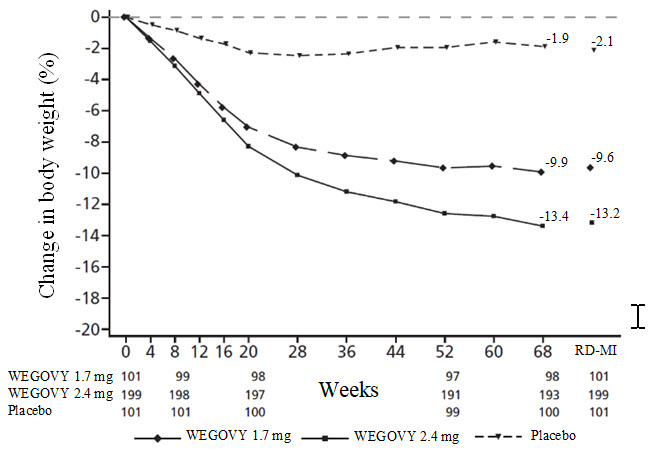
Observed values for patients completing each scheduled visit and estimates with multiple imputations from retrieved dropouts (RD-MI). At baseline, 24.7% of patients had type 2 diabetes mellitus.
Effect of WEGOVY on Anthropometry and Cardiometabolic Parameters in Adults
Changes in waist circumference and cardiometabolic parameters with WEGOVY are shown inTable 9 for Studies 2, 3, and 4; inTable 10 for Study 5; and inTable 11 for Study 6.
**Table 9. Changes in Anthropometry and Cardiometabolic Parameters at Week 68 in Studies 2, 3, and 4**
|
Study 2 (Obesity or overweight with comorbidity) |
Study 3 (Type 2 diabetes with obesity or overweight) |
Study 4 (Obesity or overweight with comorbidity undergoing intensive lifestyle therapy) | ||||
|---|---|---|---|---|---|---|
|
Intention-to-Treat |
PLACEBO |
WEGOVY |
PLACEBO |
WEGOVY |
PLACEBO |
WEGOVY |
|
Waist Circumference (cm) |
114.8 -4.1 |
114.6 -13.5 -9.4 |
115.5 -4.5 |
114.5 -9.4 -4.9 |
111.8 -6.3 |
113.6 -14.6 -8.3 |
|
Systolic Blood Pressure (mmHg) |
127 -1.1 |
126 -6.2 -5.1 |
130 -0.5 |
130 -3.9 -3.4 |
124 -1.6 |
124 -5.6 -3.9 |
|
Diastolic Blood Pressure (mmHg)2 |
80 -0.4 |
80 -2.8 -2.4 |
80 -0.9 |
80 -1.6 -0.7 |
81 -0.8 |
80 -3 -2.2 |
|
Heart Rate2,3 |
72 -0.7 |
72 3.5 4.3 |
76 -0.2 |
75 2.5 2.7 |
71 2.1 |
71 3.1 1 |
|
HbA1c (%)2 |
5.7 -0.2 |
5.7 -0.4 -0.3 |
8.1 -0.4 |
8.1 -1.6 -1.2 |
5.8 -0.3 |
5.7 -0.5 -0.2 |
|
Total Cholesterol (mg/dL)2,4 |
192.1 0.1 |
189.6 -3.3 -3.3 |
170.8 -0.5 |
170.8 -1.4 -0.9 |
188.7 2.1 |
185.4 -3.9 -5.8 |
|
LDL Cholesterol (mg/dL)2,4 |
112.5 1.3 |
110.3 -2.5 -3.8 |
90.1 0.1 |
90.1 0.5 0.4 |
111.8 2.6 |
107.7 -4.7 -7.1 |
|
HDL (mg/dL)2,4 |
49.5 1.4 |
49.4 5.2 3.8 |
43.8 4.1 |
44.7 6.9 2.7 |
50.9 5 |
51.6 6.5 1.5 |
|
Triglycerides (mg/dL)2,4 |
127.9 -7.3 |
126.2 -21.9 -15.8 |
159.5 -9.4 |
154.9 -22 -13.9 |
110.9 -6.5 |
107.9 -22.5 -17 |
Missing data were imputed from retrieved subjects of the same randomized treatment arm (RD-MI)
1Model based estimates based on an analysis of covariance model including treatment (and stratification factors for Study 3 only) as a factor and baseline value as a covariate
2Not included in the pre-specified hierarchical testing (except HbA1c for Study 3)
3Model based estimates based on a mixed model for repeated measures including treatment (and stratification factors for Study 3 only) as a factor and baseline values as a covariate
4Baseline value is the geometric mean
**Table 10. Mean Changes in Anthropometry and Cardiometabolic Parameters in Study 5 (Obesity or Overweight with Comorbidity after 20-week Run-in)****1**
|
PLACEBO N=268 |
WEGOVY N=535 | ||||
|
Randomization (week 20) |
Change from Randomization (LSMean1) |
Randomization (week 20) |
Change from Randomization (week 20) to week 68 ** (LSMean1)** |
Difference from placebo (LSMean) | |
|
Waist Circumference (cm) |
104.7 |
3.3 |
105.5 |
-6.4 |
-9.7 |
|
Systolic Blood Pressure (mmHg) |
121 |
4.4 |
121 |
0.5 |
-3.9 |
|
Diastolic Blood Pressure (mmHg)2 |
78 |
0.9 |
78 |
0.3 |
-0.5 |
|
Heart Rate2,3 |
76 |
-5.3 |
76 |
-2 |
3.3 |
|
HbA1c (%)2 |
5.4 |
0.1 |
5.4 |
-0.1 |
-0.2 |
|
Total Cholesterol (mg/dL)2,4 |
175.1 |
11.4 |
175.9 |
4.9 |
-5.8 |
|
LDL Cholesterol (mg/dL)2,4 |
109.1 |
7.6 |
108.7 |
1.1 |
-6.1 |
|
HDL Cholesterol (mg/dL)2,4 |
43.6 |
17.8 |
44.5 |
18.2 |
0.3 |
|
Triglycerides (mg/dL)2,4 |
95.3 |
14.8 |
98.1 |
-5.6 |
-17.8 |
Missing data were imputed from retrieved subjects of the same randomized treatment arm (RD-MI).
1Model based estimates based on an analysis of covariance model including treatment as a factor and baseline value as a covariate.
2Not included in the pre-specified hierarchical testing.
3Model based estimates based on a mixed model for repeated measures including treatment as a factor and baseline values as a covariate.
4Baseline value is the geometric mean.
**Table 11. Mean Changes in Anthropometry and Cardiometabolic Parameters at Week 68 in Study 6 in East-Asian Patients (WEGOVY 1.7 mg)**
|
Study 6 (BMI ≥35 kg/m2with at least one comorbidity or BMI 27 to 34.9 kg/m2with at least two comorbidities) | |||
|
Intention-to-treat |
PLACEBO N=101 |
WEGOVY 1.7 mg N=101 |
WEGOVY 2.4 mg N=199 |
|
Waist circumference (cm) Baseline Change from baseline (LSMean1) Difference from placebo (LSMean) |
103.8 -1.8 |
101.4 -7.7 -5.9 |
103.8 -11 -9.3 |
|
Systolic blood pressure (mmHg)2 Baseline Change from baseline (LSMean1) Difference from placebo (LSMean) |
133 -5.3 |
135 -10.8 -5.4 |
133 -10.8 -5.5 |
|
Diastolic blood pressure (mmHg)2 Baseline Change from baseline (LSMean1) Difference from placebo (LSMean) |
86 -2.2 |
85 -4.6 -2.4 |
83 -5.3 -3.1 |
|
Heart Rate2, 3 Baseline Change from baseline (LSMean) Difference from placebo (LSMean) |
73 2.4 |
73 4.4 2 |
73 6.3 3.9 |
|
HbA1c (%)2 Baseline Change from baseline (LSMean1) Difference from placebo (LSMean) |
6.4 0 |
6.4 -0.9 -0.9 |
6.4 -0.9 -0.9 |
|
Total Cholesterol (mg/dL)2,4 Baseline Percent change from baseline (LSMean1) Relative difference from placebo (LSMean) |
203.1 0.8 |
203.3 -6.6 -7.3 |
197.2 -8.7 -9.4 |
|
LDL Cholesterol (mg/dL)2,4 Baseline Percent change from baseline (LSMean1) Relative difference from placebo (LSMean) |
123.3 -3.8 |
120.1 -10.1 -6.5 |
116.5 -14.6 -11.2 |
|
HDL Cholesterol (mg/dL)2,4 Baseline Percent change from baseline (LSMean1) Relative difference from placebo (LSMean) |
48.7 5.9 |
50.2 6.7 0.7 |
50.8 9.2 3.1 |
|
Triglyceride (mg/dL)2,4 Baseline Percent change from baseline (LSMean1) Relative difference from placebo (LSMean) |
134.2 5.5 |
138.8 -19.5 -23.7 |
127.1 -21.2 -25.3 |
Missing data were imputed from retrieved subjects of the same randomized treatment arm (RD-MI). At baseline, 24.7% of patients had type 2 diabetes mellitus
1Model based estimates based on an analysis of covariance model including treatment and type 2 diabetes status as factors and baseline value as a covariate
2Not included in the pre-specified hierarchical testing
3Model based estimates based on a mixed model for repeated measures including treatment and type 2 diabetes status as factors and baseline values as a covariate
4Baseline value is the geometric mean
14.3 Weight Reduction and Long-Term Maintenance Study in Pediatric Patients
Aged 12 Years and Older with Obesity
Overview of Clinical Trial in Pediatric Patients
WEGOVY was evaluated in a 68-week, double-blind, randomized, parallel group,
placebo-controlled, multi-center trial in 201 pubertal pediatric patients aged
12 years and older with BMI corresponding to ≥95th percentile standardized for
age and sex (Study 7) (NCT#04102189). After a 12-week lifestyle run-in period
(including dietary recommendations and physical activity counseling), patients
were randomized 2:1 to WEGOVY once weekly or placebo once weekly. WEGOVY or
matching placebo was escalated to 2.4 mg or maximally tolerated dose during a
16-week period followed by 52 weeks on maintenance dose. Of WEGOVY-treated
patients who completed the trial, 86.7% were on the 2.4 mg dosage at the end
of the trial; for 5% of patients, 1.7 mg was the maximum tolerated dosage.
The mean age was 15 years; 38% of patients were male; 79% were White, 8% were Black or African American, 2% were Asian, and 11% were of other or unknown race; and 11% were of Hispanic or Latino ethnicity. The mean baseline body weight was 108 kg, and mean BMI was 37 kg/m2.
Results
The proportions of patients who discontinued study drug were 10% for the WEGOVY-treated group and 10% for the placebo-treated group.
The primary endpoint was percent change in BMI from baseline to week 68. After 68 weeks, treatment with WEGOVY resulted in a statistically significant reduction in percent BMI compared with placebo. Greater proportions of patients treated with WEGOVY achieved ≥5% reduction in baseline BMI than those treated with placebo as shown inTable 12.
**Table 12. Changes in Weight and BMI at Week 68 in Pediatric Patients with Obesity Aged 12 Years and Older in Study 7**
|
Intention-to-Treata |
PLACEBO |
WEGOVY |
|---|---|---|
|
BMI | ||
|
35.7 |
37.7 |
|
0.6 |
-16.1 |
|
-16.7 (-20.3; -13.2)* | |
|
% of Patients with greater than or equal to 5% reduction in baseline BMIb |
19.7 |
77.1 |
|
57.4 | |
|
% of Patients with greater than or equal to 10% reduction in baseline BMIb |
7.7 |
65.1 |
|
57.5 | |
|
% of Patients with greater than or equal to 15% reduction in baseline BMIb |
4 |
57.8 |
|
53.9 | |
|
Body Weightb | ||
|
102.6 |
109.9 |
|
2.7 |
-14.7 |
|
-17.4 |
LSMean = least squares mean; CI = confidence interval
aThe intention-to-treat population includes all randomized patients. Missing data were imputed using available data according to value and timing of last available observation on treatment and endpoint’s baseline value from retrieved subjects (RD-MI). At week 68, the BMI was missing for 2.2% and 7.5% of patients randomized to WEGOVY and placebo, respectively.
bParameters not included in the pre-specified hierarchical testing.
- p<0.0001 (unadjusted 2-sided) for superiority.
The time course of change in BMI with WEGOVY and placebo from baseline through week 68 is depicted inFigure 10. The cumulative frequency distribution of change in BMI is shown inFigure 11.
**Figure 10.**Change from Baseline (%) in BMI in Pediatric Patients with Obesity Aged 12 Years and Older in Study 7
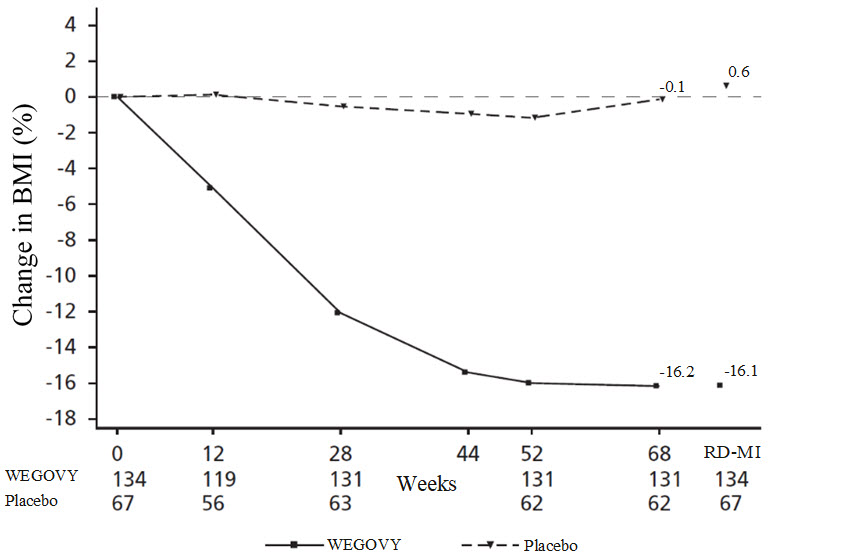
Observed values for patients completing each scheduled visit, and estimates with multiple imputations from retrieved dropouts (RD-MI)
**Figure 11.**Change in BMI (%) from Baseline to Week 68 in Pediatric Patients with Obesity Aged 12 Years and Older in Study 7
****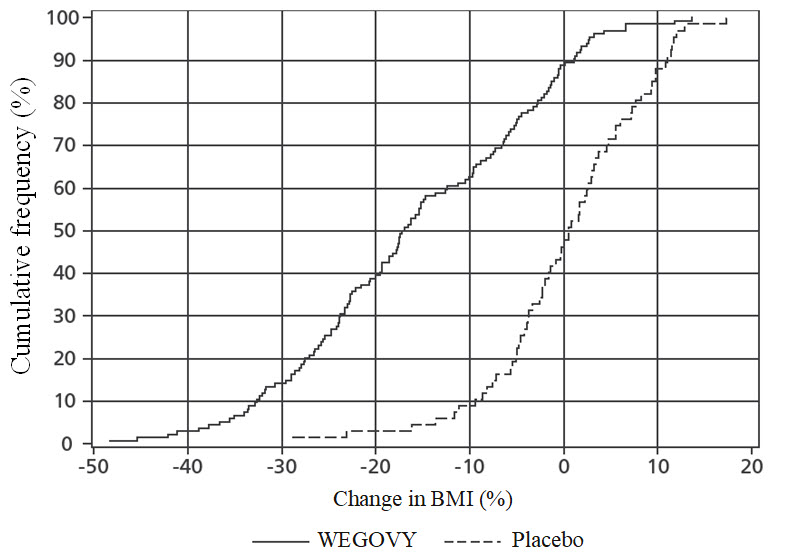
Observed data from in-trial period including imputed data for missing observations (RD-MI)
Effect of WEGOVY on Anthropometry and Cardiometabolic Parameters in Pediatric Patients with Obesity Aged 12 Years and Older
Changes in waist circumference and cardiometabolic parameters with WEGOVY are shown inTable 13 for the study in pediatric patients aged 12 years and older.
**Table 13. Mean Changes in Anthropometry and Cardiometabolic Parameters in Pediatric Patients with Obesity Aged 12 Years and Older in Study 7**
|
PLACEBO N=67 |
WEGOVY N=134 | ||||
|
Baseline |
Change from Baseline (LSMean) |
Baseline |
Change from Baseline (LSMean) |
Difference from placebo (LSMean) | |
|
Waist Circumference (cm)2 |
107.3 |
-0.6 |
111.9 |
-12.7 |
-12.1 |
|
Systolic Blood Pressure (mmHg)2 |
120 |
-0.8 |
120 |
-2.7 |
-1.9 |
|
Diastolic Blood Pressure (mmHg)2 |
73 |
-0.8 |
73 |
-1.4 |
-0.6 |
|
Heart Rate3 |
76 |
-2.3 |
79 |
1.2 |
3.5 |
|
HbA1c (%)2,4 |
5.4 |
-0.1 |
5.5 |
-0.4 |
-0.2 |
|
Total Cholesterol (mg/dL)2,5 |
160.1 |
-1.3 |
159.4 |
-8.3 |
-7.1 |
|
LDL Cholesterol (mg/dL)2,5 |
91.7 |
-3.6 |
89.8 |
-9.9 |
-6.6 |
|
HDL Cholesterol (mg/dL)2,5 |
43.3 |
3.2 |
43.7 |
8 |
4.7 |
|
Triglycerides (mg/dL)2,5 |
108 |
2.6 |
111.3 |
-28.4 |
-30.2 |
1Parameters listed in the table were not included in the pre-specified hierarchical testing.
2Missing data were imputed using available data according to value and timing of last available observation on treatment and endpoint’s baseline value from retrieved subjects (RD-MI). Model based estimates based on an analysis of covariance model including treatment and stratification groups (gender, Tanner stage group) and the interaction between stratification groups as factors and baseline value as a covariate.
3Model based estimates based on a mixed model for repeated measures including treatment as a factor and baseline value as a covariate all nested within visit.
4For patients without type 2 diabetes at randomization (N=129 for WEGOVY- treated patients and N=64 for placebo-treated patients).
5Baseline value is the geometric mean.
14.4 Noncirrhotic Metabolic Dysfunction-Associated Steatohepatitis with
Moderate to Advanced Liver Fibrosis in Adults
Overview of Clinical Trial
The efficacy of WEGOVY was evaluated based on an efficacy analysis at Week 72 in Study 8 (NCT#04822181), a 240-week, randomized, double-blind, placebo- controlled trial. Enrolled patients had a baseline or recent liver biopsy showing clinically significant MASLD (metabolic dysfunction-associated steatotic liver disease), defined as MASH with fibrosis stage 2 or 3 and a non-alcoholic fatty liver disease (NAFLD) Activity Score (NAS) ≥4 with a score of 1 or more in steatosis, lobular inflammation, and hepatocyte ballooning. Efficacy determination was based on the effect of WEGOVY on resolution of steatohepatitis without worsening of liver fibrosis and on at least one stage improvement in liver fibrosis without worsening of steatohepatitis, on post- baseline liver biopsies collected at 72 weeks.
The Week 72 analysis included 800 F2 and F3 (at eligibility) patients randomized 1:2 to receive placebo (n=266) or WEGOVY once weekly (n=534), in addition to standard of care for cardiometabolic comorbidities and healthy lifestyle counseling. WEGOVY or matching placebo was escalated to 2.4 mg once weekly during the initial 16 weeks of the treatment period. Dose escalation could be prolonged or patients could remain at a lower dose if 2.4 mg once weekly was not tolerable.
Demographic and baseline characteristics were balanced between treatment and placebo groups. Overall, the median (Q1 to Q3) age of patients at baseline was 57 (49 to 65) years, 57% were female, 18% were Hispanic, 68% were White, 27% were Asian, and 0.6% were Black or African American. Median (Q1 to Q3) body mass index (BMI) was 34 (30 to 38) kg/m2 and median (Q1 to Q3) body weight was 93 (79 to 110) kg. Baseline characteristics are presented inTable 14.
Table 14. Baseline Characteristics in Adults Patients with Noncirrhotic MASH with Stage 2 to Stage 3 Fibrosis in Study 8
|
Characteristic |
Overall (N = 800) |
|
Fibrosis stage, n (%) F2 F3 |
250 (31) 550 (69) |
|
Body Mass Index (BMI, kg/m2), n (%)a <25 25-30 30-35 ≥35 |
53 (7) 164 (21) 252 (32) 330 (41) |
|
Lean MASH, n (%)b |
22 (3) |
|
Type 2 Diabetes, n (%) |
447 (56) |
|
Hypertension, n (%) |
503 (63) |
|
Dyslipidemia, n (%) |
198 (25) |
|
Statin use, n (%) |
300 (38) |
|
Fibrosis Index Based on 4 Factors (FIB-4), Median (Q1, Q3)a |
1.6 (1.1, 2.3) |
|
Enhanced Liver Fibrosis (ELF), Median (Q1, Q3) |
9.9 (9.3, 10.5) |
a Less than 5% missingness in the variable is omitted.
b Lean MASH defined as BMI <25 kg/m2 for non-Asian patients and BMI <23 kg/m2 for Asian patients.
Among the 79% of the patients with vibration-controlled transient elastography (VCTE) at baseline, median (Q1 to Q3) VCTE was 10.9 (8.6 to 15.5) kPa, which may not be representative of the entire study population. The 21% of patients with missing VCTE at baseline had higher percentages of being female and having baseline diabetes, hypertension, and dyslipidemia.
Results
Table 15 presents the Week 72 histopathology primary endpoint results comparing WEGOVY with placebo on 1) the estimated percentage of patients with resolution of steatohepatitis and no worsening of liver fibrosis and 2) the estimated percentage of patients with at least one stage improvement in liver fibrosis and no worsening of steatohepatitis. The secondary endpoint results of the estimated percentage of patients with resolution of steatohepatitis and improvement in liver fibrosis at Week 72 are also presented. Two pathologists independently read the liver biopsies for each patient; a third pathologist performed adjudication if consensus could not be reached between the two pathologists. WEGOVY demonstrated improvement on these histopathology endpoints at Week 72 compared to placebo.
Table 15. Efficacy Results at Week 72 in Adult Patients with Noncirrhotic MASH with Stage 2 or
**Stage 3 Fibrosis in Study 8**
|
Placebo N=266 |
WEGOVY N=534 | |
|
Resolution of steatohepatitis and no worsening of liver fibrosis | ||
|
Response Rate (%) |
34 |
63 |
|
Difference in response rate vs. placebo (95% CI) |
29 (21, 36)* | |
|
Improvement in liver fibrosis and no worsening of steatohepatitis | ||
|
Response Rate (%) |
22 |
37 |
|
Difference in response rate vs. placebo (95% CI) |
14 (8, 21)* | |
|
Resolution of steatohepatitis and improvement in liver fibrosis | ||
|
Response Rate (%) |
16 |
33 |
|
Difference in response rate vs. placebo (95% CI) |
17 (10, 23)* |
- Results were statistically significant.
Endpoints were evaluated according to the MASH Clinical Research Network (CRN). Resolution of steatohepatitis is defined as a score of 0 to 1 for lobular inflammation, 0 for ballooning, and any value for steatosis. No worsening of steatohepatitis is defined as no increase from baseline in score for ballooning, lobular inflammation, or steatosis.
Estimated using pooled Mantel-Haenszel (MH) estimates stratified by baseline type 2 diabetes status (presence or absence) and baseline fibrosis stage (F2 or F3) with missing data handled by reference-based multiple imputation and 95% confidence intervals (CIs) calculated using Rubin’s rule to pool Sato’s estimate of standard errors across the imputed datasets.
Another secondary endpoint was the percent change in body weight from baseline to Week 72. Patients treated with WEGOVY (mean baseline body weight 95.4 kg) achieved an average of 10.5% weight loss from baseline at Week 72, and patients treated with placebo (mean baseline weight 97.6 kg) achieved an average of 2% weight loss from baseline at Week 72; treatment with WEGOVY resulted in an average of 8.5% greater weight loss from baseline compared to placebo (95% CI: 7.4% to 9.5%).
Starting at Week 12 and through Week 72, there was a trend of greater reductions from baseline in average ALT and AST in the WEGOVY group as compared to the placebo group.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Semaglutide is a GLP-1 analogue with 94% sequence homology to human GLP-1. Semaglutide acts as a GLP-1 receptor agonist that selectively binds to and activates the GLP-1 receptor, the target for native GLP-1.
GLP-1 is a physiological regulator of appetite and caloric intake, and the GLP-1 receptor is present in several areas of the brain involved in appetite regulation. Animal studies show that semaglutide distributed to and activated neurons in brain regions involved in regulation of food intake.
The exact mechanism of CV risk reduction has not been established.
For treatment of MASH in humans, the precise mechanism of action of semaglutide is not fully understood and may involve multiple pathways mediated by weight loss and other factors. In a mouse model of diet-induced MASH, treatment with semaglutide resulted in histological improvements in steatosis, inflammation, and fibrosis in liver compared to baseline, which was associated with body weight loss, intermittent periods of reduced food intake, and improvements in relevant biomarkers. The relationship between the pathophysiology of MASH in animal models and humans has not been fully established.
12.2 Pharmacodynamics
Semaglutide lowers body weight with greater fat mass loss than lean mass loss. Semaglutide decreases calorie intake. The effects are likely mediated by affecting appetite.
Semaglutide stimulates insulin secretion and reduces glucagon secretion in a glucose-dependent manner. These effects can lead to a reduction of blood glucose.
Gastric Emptying
Semaglutide delays gastric emptying.
Cardiac Electrophysiology (QTc)
The effect of semaglutide on cardiac repolarization was tested in a thorough QTc trial. Semaglutide did not prolong QTc intervals at doses up to 1.5 mg at steady state.
Noninvasive Liver Disease Markers
Semaglutide decreases liver fat content measured by Magnetic Resonance Imaging Proton Density Fat Fraction (MRI-PDFF), liver stiffness assessed by transient elastography (TE), Enhanced Liver Fibrosis (ELF) score, and the levels of the pro-peptide of type III collagen biomarker (Pro-C3). The clinical relevance of these changes is yet to be confirmed.
12.3 Pharmacokinetics
Absorption
Absolute bioavailability of semaglutide is 89%. Maximum concentration of semaglutide is reached 1 to 3 days post dose.
Similar exposure was achieved with subcutaneous administration of semaglutide in the abdomen, thigh, or upper arm.
The average semaglutide steady state concentration following subcutaneous administration of WEGOVY was approximately 75 nmol/L in patients with either obesity (BMI greater than or equal to 30 kg/m2) or overweight (BMI greater than or equal to 27 kg/m2). The steady state exposure of WEGOVY increased proportionally with doses up to 2.4 mg once weekly.
Distribution
The mean volume of distribution of semaglutide following subcutaneous administration in patients with obesity or overweight is approximately 12.5 L. Semaglutide is extensively bound to plasma albumin (greater than 99%) which results in decreased renal clearance and protection from degradation.
Elimination
The apparent clearance of semaglutide in patients with obesity or overweight is approximately 0.05 L/h. With an elimination half-life of approximately 1 week, semaglutide will be present in the circulation for about 5 to 7 weeks after the last dose of 2.4 mg.
Metabolism
The primary route of elimination for semaglutide is metabolism following proteolytic cleavage of the peptide backbone and sequential beta-oxidation of the fatty acid sidechain.
Excretion
The primary excretion routes of semaglutide-related material are via the urine and feces. Approximately 3% of the dose is excreted in the urine as intact semaglutide.
Specific Populations
The effects of intrinsic factors on the pharmacokinetics of semaglutide are shown inFigure 2. Fibrosis stage (F2 or F3) did not impact semaglutide exposure in patients with MASH.
**Figure 2. Impact of intrinsic factors on semaglutide exposure**
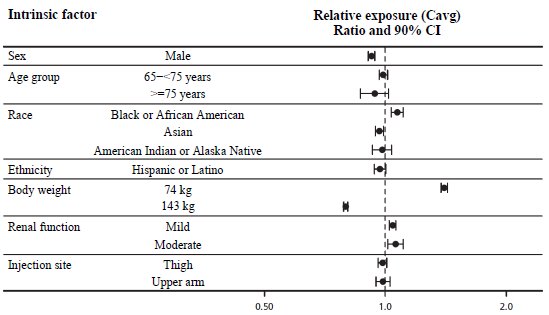
Data are steady-state dose-normalized average semaglutide exposures relative to a reference subject profile (non-Hispanic or Latino ethnicity, white female aged 18 to less than 65 years, with a body weight of 110 kg and normal renal function, who injected in the abdomen). Body weight categories (74 and 143 kg) represent the 5% and 95% percentiles in the dataset.
There is no difference observed in the exposure of semaglutide following subcutaneous administration of WEGOVY between patients with MASH and patients with overweight or obesity.
Patients with Renal Impairment
Renal impairment did not impact the exposure of semaglutide in a clinically relevant manner. The pharmacokinetics of semaglutide were evaluated following a single dose of 0.5 mg semaglutide in a study of patients with different degrees of renal impairment (mild, moderate, severe, or ESRD) compared with subjects with normal renal function. The pharmacokinetics were also assessed in subjects with overweight (BMI 27 to 29.9 kg/m2) or obesity (BMI greater than or equal to 30 kg/m2) and mild to moderate renal impairment, based on data from clinical trials.
Patients with Hepatic Impairment
Hepatic impairment did not impact the exposure of semaglutide. The pharmacokinetics of semaglutide were evaluated following a single dose of 0.5 mg semaglutide in a study of patients with different degrees of hepatic impairment (mild, moderate, severe) compared with subjects with normal hepatic function.
Drug Interactions Studies
In vitro studies have shown very low potential for semaglutide to inhibit or induce CYP enzymes, or to inhibit drug transporters.
The delay of gastric emptying with semaglutide may influence the absorption of concomitantly administered oral medications [see Drug Interactions (7.2)]. The potential effect of semaglutide on the absorption of co-administered oral medications was studied in trials at semaglutide 1 mg steady-state exposure. No clinically relevant drug-drug interactions with semaglutide (Figure 3) were observed based on the evaluated medications. In a separate study, no apparent effect on the rate of gastric emptying was observed with semaglutide 2.4 mg.
**Figure 3. Impact of semaglutide 1 mg on the pharmacokinetics of co-administered medications**
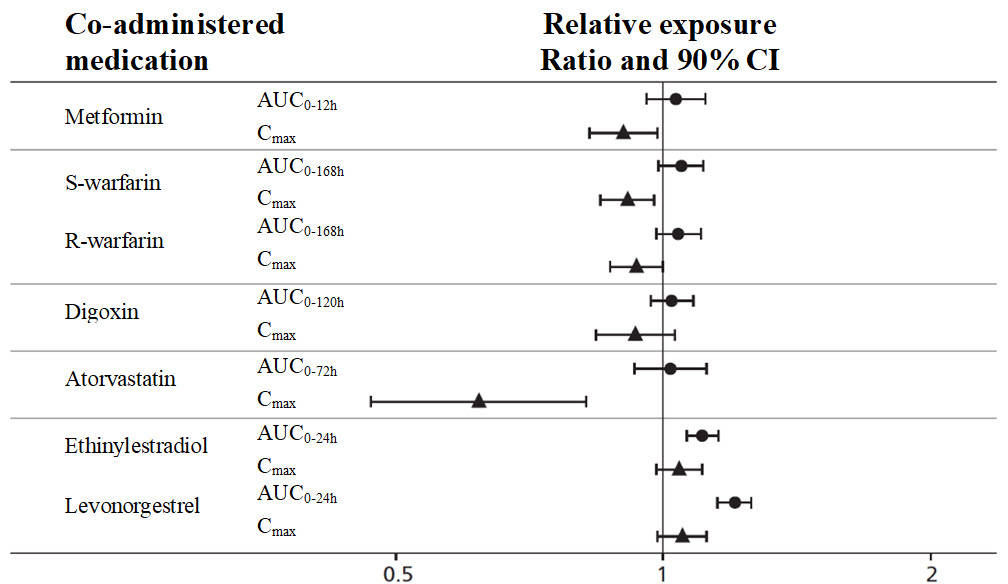
Relative exposure in terms of AUC and Cmax for each medication when given with semaglutide compared to without semaglutide. Metformin and oral contraceptive drug (ethinylestradiol/levonorgestrel) were assessed at steady state. Warfarin (S-warfarin/R-warfarin), digoxin and atorvastatin were assessed after a single dose.
Abbreviations: AUC: area under the curve, Cmax: maximum concentration, CI: confidence interval.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of semaglutide or of other semaglutide products.
During the 68-week treatment periods in Studies 2 and 3 [see Clinical Studies (14.2)], 50/1709 (3%) of WEGOVY-treated patients developed anti-semaglutide antibodies. Of these 50 WEGOVY-treated patients, 28 patients (2% of the total WEGOVY-treated study population) developed antibodies that cross-reacted with native GLP-1. In the patients with MASH treated with WEGOVY for 72 weeks in Study 8 [see Clinical Studies (14.4)], 3/763 (0.4%) of patients developed anti-semaglutide antibodies which were also cross-reactive to native GLP-1. No identified clinically significant effect of anti-semaglutide antibodies on pharmacokinetics for WEGOVY was observed. There is insufficient evidence to characterize the effects of anti-semaglutide antibodies on pharmacodynamics, safety, or effectiveness of semaglutide products.
SPL MEDGUIDE SECTION
Medication Guide
|
Medication Guide WEGOVY**®**** (wee-GOH-vee)** (semaglutide) injection, for subcutaneous use | |||
|
Read this Medication Guide and Instructions for Use before you start using WEGOVY and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. | |||
|
What is the most important information I should know about WEGOVY? WEGOVY may cause serious side effects, including: • • | |||
|
What is WEGOVY? • o o o • • • o o o | |||
|
Do not use WEGOVY if: • • | |||
|
Before using WEGOVY, tell your healthcare provider if you have any other medical conditions, including if you: • • • • • o • Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. | |||
|
How should I use WEGOVY? • • • • • • • • • • • • | |||
|
What are the possible side effects of WEGOVY? WEGOVY may cause serious side effects, including: • • • | |||
|
o |
o | ||
|
o |
o | ||
|
• | |||
|
o |
o |
o | |
|
o |
o |
o | |
|
o |
o |
o | |
|
o |
o | ||
|
o |
o | ||
|
• • • | |||
|
o |
o |
o | |
|
o |
o | ||
|
• • • • The most common side effects of WEGOVY in adults or children aged 12 years and older may include: | |||
|
• |
• |
• |
• |
|
• |
• |
• |
• |
|
• |
• |
• |
• |
|
• |
• |
• |
• |
|
Talk to your healthcare provider about any side effect that bothers you or does not go away. These are not all the possible side effects of WEGOVY. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088. | |||
|
General information about the safe and effective use of WEGOVY. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use WEGOVY for a condition for which it was not prescribed. Do not give WEGOVY to other people, even if they have the same condition that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about WEGOVY that is written for health professionals. | |||
|
What are the ingredients in WEGOVY? Active Ingredient: semaglutide **Inactive Ingredients:**disodium phosphate dihydrate, 1.42 mg; sodium chloride, 8.25 mg; water for injection, and hydrochloric acid or sodium hydroxide may be added to adjust pH. | |||
|
Manufactured by: Novo Nordisk A/S, DK-2880 Bagsvaerd, Denmark WEGOVY® is a registered trademark of Novo Nordisk A/S. PATENT Information: http://novonordisk-us.com/products/product-patents.html © 2025 Novo Nordisk For more information, go to startWegovy.com or call 1-833-Wegovy-1. |
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 08/2025
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Indications and Usage (1)……………………………………..…….08/2025
Dosage and Administration, Recommended Dosage Initiation and Escalation Schedule, Recommended Maintenance Dosage (2.2, 2.3)……………………… 08/2025
Warnings and Precautions, Acute Kidney Injury Due to Volume Depletion (5.5)………………..…..08/2025
Warning and Precautions, Severe Gastrointestinal Adverse Reactions, Pulmonary Aspiration During General Anesthesia or Deep Sedation (5.6, 5.11)…………………………………………………..… 11/2024
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Monitoring and Administration Instructions
•
In patients with type 2 diabetes mellitus, monitor blood glucose prior to starting WEGOVY and during WEGOVY treatment [see Warnings and Precautions (5.4)].
•
Prior to initiation of WEGOVY, train patients on proper injection technique. Refer to the accompanying Instructions for Use for complete administration instructions with illustrations.
•
Inspect WEGOVY visually prior to each injection. Only use if solution is clear, colorless, and contains no particles.
•
Administer WEGOVY in combination with a reduced-calorie diet and increased physical activity.
•
Administer WEGOVY once weekly, on the same day each week, at any time of day, with or without meals.
•
Inject WEGOVY subcutaneously in the abdomen, thigh, or upper arm. The time of day and the injection site can be changed without dose adjustment.
2.2 Recommended Dosage Initiation and Escalation Schedule
•
Initiate WEGOVY with a dosage of 0.25 mg injected subcutaneously once weekly. Follow the recommended dosage initiation and escalation in**Table 1** for all indications to reduce the risk of gastrointestinal adverse reactions [see Warnings and Precautions (5.6), Adverse Reactions (6.1)].
•
If patients do not tolerate a dose during dosage escalation, consider delaying dosage escalation for 4 weeks.
Table 1. Recommended Dosage Initiation and Escalation
|
Treatment |
Weeks |
Once Weekly Subcutaneous Dosage |
|
Initiation |
1 through 4 |
0.25 mg |
|
Escalation |
5 through 8 |
0.5 mg |
|
9 through 12 |
1 mg | |
|
13 through 16 |
1.7 mg | |
|
Maintenance |
17 and onward |
See indication below (Subsection 2.3) for maintenance dosage |
2.3 Recommended Maintenance Dosage
Cardiovascular Risk Reduction and Weight Reduction
•
The maintenance dosage of WEGOVY for CV risk reduction and weight reduction is either 2.4 mg (recommended) or 1.7 mg injected subcutaneously once weekly.
•
Consider treatment response and tolerability when selecting the maintenance dosage [see Adverse Reactions (6.1), Clinical Studies (14.2, 14.3)].
Noncirrhotic MASH with Moderate to Advanced Liver Fibrosis
•
The recommended maintenance dosage of WEGOVY for the treatment of noncirrhotic MASH with moderate to advanced liver fibrosis is 2.4 mg injected subcutaneously once weekly.
•
If patients do not tolerate the maintenance dosage of 2.4 mg once weekly, the dosage can be decreased to 1.7 mg once weekly. Consider reescalation to 2.4 mg once weekly [see Adverse Reactions (6.1), Clinical Studies (14.4)].
2.4 Recommendations Regarding Missed Dose
•
If one dose is missed and the next scheduled dose is more than 2 days away (48 hours), administer WEGOVY as soon as possible. If one dose is missed and the next scheduled dose is less than 2 days away (48 hours), do not administer the dose. Resume dosing on the regularly scheduled day of the week.
•
If 2 or more consecutive doses are missed, resume dosing as scheduled or, if needed, reinitiate WEGOVY and follow the dose escalation schedule, which may reduce the occurrence of gastrointestinal symptoms associated with reinitiation of treatment.
•
Administer WEGOVY once weekly as an adjunct to diet and increased physical activity, on the same day each week, at any time of day, with or without meals (2.1).
•
Inject subcutaneously in the abdomen, thigh, or upper arm (2.1).
•
In patients with type 2 diabetes, monitor blood glucose prior to starting and during WEGOVY treatment (2.1).
•
Initiate at 0.25 mg once weekly for 4 weeks. Then follow the dosage escalation schedule, titrating every 4 weeks to achieve the maintenance dosage (2.2).
•
For patients with MASH, the maintenance dosage of WEGOVY is 2.4 mg once weekly. If patients do not tolerate 2.4 mg once weekly, the dosage can be decreased to 1.7 mg once weekly. (2.3)
•
For all other indications,except for MASH treatment, the maintenance dosage of WEGOVY is either 2.4 mg (recommended) or 1.7 mg once weekly. (2.3)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Injection: clear, colorless solution available in 5 prefilled, disposable, single-dose pens:
•
0.25 mg/0.5 mL
•
0.5 mg/0.5 mL
•
1 mg/0.5 mL
•
1.7 mg/0.75 mL
•
2.4 mg/0.75 mL
Injection: prefilled, single-dose pen that delivers doses of 0.25 mg, 0.5 mg, 1 mg, 1.7 mg or 2.4 mg. (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to semaglutide during pregnancy. Pregnant women exposed to WEGOVY and healthcare providers are encouraged to contact Novo Nordisk at 1-877-390-2760 or www.wegovypregnancyregistry.com.
Risk Summary
Based on animal reproduction studies, there may be potential risks to the fetus from exposure to semaglutide during pregnancy. Available pharmacovigilance data and data from clinical trials with WEGOVY use in pregnant patients are insufficient to establish a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes.
Weight loss offers no benefit to a pregnant patient and may cause fetal harm. When a pregnancy is recognized, advise the pregnant patient of the risk to a fetus, and discontinue WEGOVY (see Clinical Considerations).
There may be risks to the mother and fetus related to underlying MASH with advanced liver fibrosis (see Clinical Considerations). Whether semaglutide treatment during pregnancy reduces these risks is unknown. WEGOVY for the treatment of MASH should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In pregnant rats administered semaglutide during organogenesis, embryofetal mortality, structural abnormalities and alterations to growth occurred at maternal exposures below the maximum recommended human dose (MRHD) based on AUC. In rabbits and cynomolgus monkeys administered semaglutide during organogenesis, early pregnancy losses and structural abnormalities were observed at below the MRHD (rabbit) and greater than or equal to 2-fold the MRHD (monkey). These findings coincided with a marked maternal body weight loss in both animal species (see Data).
The background risk of major birth defects and miscarriage for the indicated populations are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Appropriate weight gain based on pre-pregnancy weight is currently recommended for all pregnant patients, including those who already have overweight or obesity, because of the obligatory weight gain that occurs in maternal tissues during pregnancy.
There may be risks to the mother and fetus related to MASH with advanced liver fibrosis, such as increased risks of gestational diabetes, hypertensive complications, preterm birth, and postpartum hemorrhage. The effect of semaglutide on these risks is unknown.
Data
Animal Data
In a combined fertility and embryofetal development study in rats, subcutaneous doses of 0.01, 0.03, and 0.09 mg/kg/day (0.04-, 0.1-, and 0.4-fold the MRHD) were administered to males for 4 weeks prior to and throughout mating and to females for 2 weeks prior to mating, and throughout organogenesis to Gestation Day 17. In parental animals, pharmacologically mediated reductions in body weight gain and food consumption were observed at all dose levels. In the offspring, reduced growth and fetuses with visceral (heart blood vessels) and skeletal (cranial bones, vertebra, ribs) abnormalities were observed at the human exposure.
In an embryofetal development study in pregnant rabbits, subcutaneous doses of 0.001, 0.0025, or 0.0075 mg/kg/day (0.01-, 0.1-, and 0.9-fold the MRHD) were administered throughout organogenesis from Gestation Day 6 to 19. Pharmacologically mediated reductions in maternal body weight gain and food consumption were observed at all dose levels. Early pregnancy losses and increased incidences of minor visceral (kidney, liver) and skeletal (sternebra) fetal abnormalities were observed at greater than or equal to 0.0025 mg/kg/day, at clinically relevant exposures.
In an embryofetal development study in pregnant cynomolgus monkeys, subcutaneous doses of 0.015, 0.075, and 0.15 mg/kg twice weekly (0.4-, 2-, and 6-fold the MRHD) were administered throughout organogenesis, from Gestation Day 16 to 50. Pharmacologically mediated, marked initial maternal body weight loss and reductions in body weight gain and food consumption coincided with the occurrence of sporadic abnormalities (vertebra, sternebra, ribs) at greater than or equal to 0.075 mg/kg twice weekly (greater than or equal to 2 times human exposure).
In a pre- and postnatal development study in pregnant cynomolgus monkeys, subcutaneous doses of 0.015, 0.075, and 0.15 mg/kg twice weekly (0.2-, 1-, and 3-fold the MRHD) were administered from Gestation Day 16 to 140. Pharmacologically mediated marked initial maternal body weight loss and reductions in body weight gain and food consumption coincided with an increase in early pregnancy losses and led to delivery of slightly smaller offspring at greater than or equal to 0.075 mg/kg twice weekly (greater than or equal to 1-time human exposure).
8.2 Lactation
Risk Summary
There are no data on the presence of subcutaneously administered semaglutide or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. Semaglutide was present in the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for WEGOVY and any potential adverse effects on the breastfed infant from WEGOVY or from the underlying maternal condition.
Data
In lactating rats, semaglutide was detected in milk at levels 3- to 12-fold lower than in maternal plasma.
8.3 Females and Males of Reproductive Potential
Based on data from animal reproduction studies, females of reproductive potential receiving WEGOVY for CV risk reduction or weight reduction or females of reproductive potential receiving WEGOVY for MASH in whom the potential risk outweighs the potential benefit should discontinue WEGOVY at least 2 months before they plan to become pregnant to account for the long half-life of semaglutide [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and effectiveness of WEGOVY in combination with a reduced calorie diet and increased physical activity to reduce excess body weight and maintain weight reduction long term in pediatric patients aged 12 years and older with obesity have been established. Use of WEGOVY for this indication is supported by a 68-week, double-blind, placebo-controlled clinical trial in 201 pediatric patients aged 12 years and older with a BMI corresponding to ≥95th percentile for age and sex [see Clinical Studies (14.3)] and from trials in adult patients with obesity [see Clinical Studies (14.2)]. Use of the 1.7 mg once weekly maintenance dosage of WEGOVY in pediatric patients is also supported by additional exposure-efficacy and safety analyses in pooled adult and pediatric patients.
Adverse reactions with WEGOVY treatment in pediatric patients aged 12 years and older were generally similar to those reported in adults. Pediatric patients aged 12 years and older treated with WEGOVY had greater incidences of cholelithiasis, cholecystitis, hypotension, rash, and urticaria compared to adults treated with WEGOVY [see Adverse Reactions (6.1)].
There are insufficient data in pediatric patients with type 2 diabetes treated with WEGOVY for obesity to determine if there is an increased risk of hypoglycemia with WEGOVY treatment similar to that reported in adults. Inform patients of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia. In pediatric patients aged 12 years and older with type 2 diabetes, monitor blood glucose prior to starting WEGOVY and during WEGOVY treatment. When initiating WEGOVY in pediatric patients aged 12 years and older with type 2 diabetes, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia [see Warnings and Precautions (5.4)].
The safety and effectiveness of WEGOVY have not been established in pediatric patients:
•
To reduce the risk of major adverse CV events. Clinical trials for this indication are highly impracticable because of the low prevalence of the condition in pediatric patients.
•
To reduce excess body weight and maintain weight reduction long term in those less than 12 years of age.
•
For the treatment of noncirrhotic MASH.
8.5 Geriatric Use
In the WEGOVY clinical trials for weight reduction and long-term maintenance, 233 (9%) WEGOVY-treated patients were aged 65 to 75 years and 23 (1%) WEGOVY- treated patients were aged 75 years and older [see Clinical Studies (14.2)]. In a CV outcomes trial, 2,656 (30%) WEGOVY-treated patients were aged 65 to 75 years and 703 (8%) WEGOVY-treated patients were aged 75 years and older [see Clinical Studies (14.1)]. No overall difference in effectiveness was observed between patients aged 65 years and older and younger adult patients. In the CV outcomes trial, patients aged 75 years and older reported more fractures of the hip and pelvis on WEGOVY than on placebo. Patients aged 75 years and older (WEGOVY-treated and placebo-treated) reported more serious adverse reactions overall compared to younger adult patients [see Adverse Reactions (6.1)].
In the clinical trial in patients with MASH, of the 534 patients randomized to WEGOVY, 138 (26%) were aged 65 years and older and 13 (2%) were aged 75 years and older [see Clinical Studies (14.4)]. No overall differences in safety or effectiveness of WEGOVY have been observed between patients 65 years of age and older and younger adult patients.
8.6 Renal Impairment
The recommended dosage of WEGOVY in patients with renal impairment is the same as those with normal renal function. In a study in patients with renal impairment, including end-stage renal disease, no clinically relevant change in semaglutide pharmacokinetics was observed [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
The recommended dosage of WEGOVY in patients with hepatic impairment is the same as those with normal hepatic function. In a study in patients with different degrees of hepatic impairment, no clinically relevant change in semaglutide pharmacokinetics was observed [see Clinical Pharmacology (12.3)].
•
Pregnancy: May cause fetal harm. For patients receiving WEGOVY for CV risk reduction or weight reduction, discontinue WEGOVY when pregnancy is recognized. For patients with MASH, use during pregnancy only if the potential benefit justifies the potential risk to the fetus. (8.1)
•
Females and Males of Reproductive Potential: For patients receiving WEGOVY for CV risk reduction or weight reduction, or for MASH where the potential risk outweighs the potential benefit, discontinue WEGOVY at least 2 months before a planned pregnancy because of the long half-life of semaglutide. (8.3)
OVERDOSAGE SECTION
10 OVERDOSAGE
Overdoses have been reported with other GLP-1 receptor agonists. Effects have included severe nausea, severe vomiting, and severe hypoglycemia. In the event of overdose, appropriate supportive treatment should be initiated according to the patient’s clinical signs and symptoms. In the event of an overdose of WEGOVY, consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations. A prolonged period of observation and treatment for these symptoms may be necessary, taking into account the long half-life of WEGOVY of approximately 1 week.
DESCRIPTION SECTION
11 DESCRIPTION
WEGOVY (semaglutide) injection, for subcutaneous use, contains semaglutide, a human GLP-1 receptor agonist (or GLP-1 analog). The peptide backbone is produced by yeast fermentation. The main protraction mechanism of semaglutide is albumin binding, facilitated by modification of position 26 lysine with a hydrophilic spacer and a C18 fatty di-acid. Furthermore, semaglutide is modified in position 8 to provide stabilization against degradation by the enzyme dipeptidyl-peptidase 4 (DPP-4). A minor modification was made in position 34 to ensure the attachment of only one fatty di-acid. The molecular formula is C187H291N45O59 and the molecular weight is 4113.58 g/mol.
**Figure 1. Structural Formula of Semaglutide**
WEGOVY is a sterile, aqueous, clear, colorless solution. Each 0.5 mL single- dose pen contains a solution of WEGOVY containing 0.25 mg, 0.5 mg or 1 mg of semaglutide; and each 0.75 mL single-dose pen contains a solution of WEGOVY containing 1.7 or 2.4 mg of semaglutide. Each 1 mL of WEGOVY contains the following inactive ingredients: disodium phosphate dihydrate, 1.42 mg; sodium chloride, 8.25 mg; and water for injection. WEGOVY has a pH of approximately 7.4. Hydrochloric acid or sodium hydroxide may be added to adjust pH.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year carcinogenicity study in CD-1 mice, subcutaneous doses of 0.3, 1, and 3 mg/kg/day (2-, 8-, and 22-fold the maximum recommended human dose [MRHD] of 2.4 mg/week, based on AUC) were administered to the males, and 0.1, 0.3, and 1 mg/kg/day (0.6-, 2-, and 5-fold MRHD) were administered to the females. A statistically significant increase in thyroid C-cell adenomas and a numerical increase in C-cell carcinomas were observed in males and females at all dose levels (greater than or equal to 0.6 times human exposure).
In a 2-year carcinogenicity study in Sprague Dawley rats, subcutaneous doses of 0.0025, 0.01, 0.025 and 0.1 mg/kg/day were administered (below quantification, 0.2-, 0.4-, and 2-fold the exposure at the MRHD). A statistically significant increase in thyroid C-cell adenomas was observed in males and females at all dose levels, and a statistically significant increase in thyroid C-cell carcinomas was observed in males at greater than or equal to 0.01 mg/kg/day, at clinically relevant exposures.
Human relevance of thyroid C-cell tumors in rats is unknown and could not be determined by clinical studies or nonclinical studies [see Boxed Warning, Warnings and Precautions (5.1)]. Semaglutide was not mutagenic or clastogenic in a standard battery of genotoxicity tests (bacterial mutagenicity [Ames] human lymphocyte chromosome aberration, rat bone marrow micronucleus).
In a combined fertility and embryo-fetal development study in rats, subcutaneous doses of 0.01, 0.03, and 0.09 mg/kg/day (0.04-, 0.1-, and 0.4-fold the MRHD) were administered to male and female rats. Males were dosed for 4 weeks prior to mating, and females were dosed for 2 weeks prior to mating and throughout organogenesis until Gestation Day 17. No effects were observed on male fertility. In females, an increase in estrus cycle length was observed at all dose levels, together with a small reduction in numbers of corpora lutea at greater than or equal to 0.03 mg/kg/day. These effects were likely an adaptive response secondary to the pharmacological effect of semaglutide on food consumption and body weight.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
WEGOVY injection is a clear, colorless solution in a prefilled, disposable, single-dose pen-injector with an integrated needle. It is supplied in cartons containing 4 pen-injectors in the following packaging configurations:
|
Total Strength per Total Volume |
NDC |
|
0.25 mg/0.5 mL |
0169-4525-14 |
|
0.5 mg/0.5 mL |
0169-4505-14 |
|
1 mg/0.5 mL |
0169-4501-14 |
|
1.7 mg/0.75 mL |
0169-4517-14 |
|
2.4 mg/0.75 mL |
0169-4524-14 |
Storage and Handling
Store the WEGOVY single-dose pen in the refrigerator from 2°C to 8°C (36°F to 46°F). If needed, prior to cap removal, the pen can be kept from 8°C to 30°C (46°F to 86°F) up to 28 days. Do not freeze. Protect WEGOVY from light. WEGOVY must be kept in the original carton until time of administration. Discard the WEGOVY pen after use.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Risk of Thyroid C-cell Tumors
Inform patients that semaglutide causes thyroid C-cell tumors in rodents and that the human relevance of this finding has not been determined. Counsel patients to report symptoms of thyroid tumors (e.g., a lump in the neck, hoarseness, dysphagia, or dyspnea) to their physician [see Boxed Warning, Warnings and Precautions (5.1)].
Acute Pancreatitis
Inform patients of the potential risk for acute pancreatitis and its symptoms: severe abdominal pain that sometimes radiates to the back, and which may or may not be accompanied by nausea or vomiting. Instruct patients to discontinue WEGOVY promptly and contact their physician if pancreatitis is suspected [see Warnings and Precautions (5.2)].
Acute Gallbladder Disease
Inform patients of the risk of acute gallbladder disease. Advise patients that substantial or rapid weight loss can increase the risk of gallbladder disease, but that gallbladder disease may also occur in the absence of substantial or rapid weight loss. Instruct patients to contact their healthcare provider for appropriate clinical follow-up if gallbladder disease is suspected [see Warnings and Precautions (5.3)].
Hypoglycemia
Inform patients of the risk of hypoglycemia and educate patients on the signs and symptoms of hypoglycemia. Advise patients with diabetes mellitus on glycemic lowering therapy that they may have an increased risk of hypoglycemia when using WEGOVY and to report signs and/or symptoms of hypoglycemia to their healthcare provider [see Warnings and Precautions (5.4)].
Acute Kidney Injury Due to Volume Depletion
Inform patients of the potential risk of acute kidney injury due to dehydration associated with gastrointestinal adverse reactions. Advise patients to take precautions to avoid fluid depletion. Inform patients of the signs and symptoms of acute kidney injury and instruct them to promptly report any of these signs or symptoms or persistent (or extended) nausea, vomiting, and diarrhea to their healthcare provider [see Warnings and Precautions (5.5)].
Severe Gastrointestinal Adverse Reactions
Inform patients of the potential risk of severe gastrointestinal adverse reactions. Instruct patients to contact their healthcare provider if they have severe or persistent gastrointestinal symptoms [see Warnings and Precautions (5.6)].
Hypersensitivity Reactions
Inform patients that serious hypersensitivity reactions have been reported during postmarketing use of semaglutide, the active ingredient in WEGOVY. Advise patients on the symptoms of hypersensitivity reactions and instruct them to stop taking WEGOVY and seek medical advice promptly if such symptoms occur [see Warnings and Precautions (5.7)].
Diabetic Retinopathy Complications in Patients with Type 2 Diabetes
Inform patients with type 2 diabetes to contact their physician if changes in vision are experienced during treatment with WEGOVY [see Warnings and Precautions (5.8)].
Heart Rate Increase
Instruct patients to inform their healthcare providers of palpitations or feelings of a racing heartbeat while at rest during WEGOVY treatment [see Warnings and Precautions (5.9)].
Suicidal Behavior and Ideation
Advise patients to report emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Inform patients that if they experience suicidal thoughts or behaviors, they should stop taking WEGOVY [see Warnings and Precautions (5.10)].
Pulmonary Aspiration During General Anesthesia or Deep Sedation
Inform patients that WEGOVY may cause their stomach to empty more slowly which may lead to complications with anesthesia or deep sedation during planned surgeries or procedures. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking WEGOVY [see Warnings and Precautions (5.11)].
Pregnancy
WEGOVY may cause fetal harm. Advise patients to inform their healthcare provider of a known or suspected pregnancy. Advise patients who are exposed to WEGOVY during pregnancy to contact Novo Nordisk at 1-877-390-2760 or www.wegovypregnancyregistry.com [see Use in Specific Populations (8.1)].
Missed Doses
Inform patients if a dose is missed and the next scheduled dose is more than 2 days away (48 hours), administer WEGOVY as soon as possible. If one dose is missed and the next scheduled dose is less than 2 days away (48 hours), do not administer the dose. Inform patients to resume their regular once weekly dosing schedule [see Dosage and Administration (2.4)].
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd
Denmark
For additional information about WEGOVY contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-833-934-6891
WEGOVY® is a registered trademark of Novo Nordisk A/S.
PATENT INFORMATION: http://www.novonordisk-us.com/products/product- patents.html
© 2025 Novo Nordisk
INSTRUCTIONS FOR USE SECTION
Instructions for Use
|
Instructions for Use WEGOVY**®** (semaglutide) injection WEGOVY comes in five strengths:
Before you use your WEGOVY pen for the first time, talk to your healthcare provider or your caregiver about how to prepare and inject WEGOVY correctly. |
|
|
Important information Read this Instructions for Use before you start using WEGOVY. This information does not replace talking to your healthcare provider about your medical condition or treatment. ·Your WEGOVY pen is for 1 time use only. The WEGOVY pen is for subcutaneous (under the skin) use only. ·The dose of WEGOVY is already set on your pen. ·The needle is covered by the needle cover and the needle will not be seen. · Do not remove the pen cap until you are ready to inject. · Do not touch or push on the needle cover. You could get a needle stick injury. · Your WEGOVY injection will start when the needle cover is pressed firmly against your skin. ·Do not remove the pen from your skin before the yellow bar in the pen window has stopped moving. The medicine may appear on the skin or squirt from the needle and you may not get your full dose of WEGOVY if: • • · If the yellow bar does not start moving or stops during the injection, contact your healthcare provider or Novo Nordisk at startWegovy.com or call Novo Nordisk Inc. at 1-833-934-6891. · The needle cover will lock when the pen is removed from your skin.You cannot stop the injection and restart it later. · People who are blind or have vision problems should not use the WEGOVY pen without help from a person trained to use the WEGOVY pen. | |
|
How do I store WEGOVY? · Store the WEGOVY pen in the refrigerator between 36°F to 46°F (2°C to 8°C). · If needed, before removing the pen cap, WEGOVY can be stored from 46°F to 86°F (8°C to 30°C) in the original carton for up to 28 days. · Keep WEGOVY in the original carton to protect it from light. ·Do not freeze. · Throw away the pen if WEGOVY has been frozen, has been exposed to light or temperatures above 86°F (30°C), or has been out of the refrigerator for 28 days or longer. Keep WEGOVY and all medicines out of the reach of children. | |
|
| |
|
How to use your WEGOVY pen |
Do not use your WEGOVY pen without receiving training from your healthcare provider. Make sure that you or your caregiver know how to give an injection with the pen before you start your treatment. |
|
Read and follow the instructions so that you use your WEGOVY pen correctly: Preparation Step 1. Prepare for your injection. • o o o o
• • o o o Contact Novo Nordisk at 1-833-934-6891 if your WEGOVY pen fails any of these checks. | |
|
Step 2. Choose your injection site. • Your healthcare provider can help you choose the injection site that is best for you • • Do not inject into an area where the skin is tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks. • You may inject in the same body area each week, but make sure it is not in the same spot each time. Clean the injection site with an alcohol swab or soap and water. Do not touch the injection site after cleaning. Allow the skin to dry before injecting.
| |
|
Injection Step 3. Remove pen cap. •Pull the pen cap straight off your pen. Step 4. Inject WEGOVY. • Push the pen firmly against your skin and keep applying pressure until the yellow bar ** has stopped moving.** If the yellow bar does not start moving, press the pen more firmly against your skin. • • • •
| |
|
Throw away pen Step 5. Throw away (dispose of) pen. Safely dispose of the WEGOVY pen right away after each use. See**“How do I throw away (dispose of) WEGOVY pens?”** •What if blood appears after injection? If blood appears at the injection site, press the site lightly with a gauze pad or cotton ball.
• • | |
|
How do I throw away (dispose of) WEGOVY pens? Put the used WEGOVY pen in an FDA-cleared sharps disposal container right away after use.Do not throw away (dispose of) the pen in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is: When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific sharps disposal in the state that you live in, go to the FDA’s website at http://www.fda.gov/safesharpsdisposal. Important: Keep your WEGOVY pen, sharps disposal container and all medicines out of the reach of children. |
Protect your pen |
|
Manufactured by: Novo Nordisk A/S Novo Allé DK-2880 Bagsvaerd, Denmark For information about WEGOVY, go to startWegovy.com or contact: Novo Nordisk Inc. 800 Scudders Mill Road Plainsboro, NJ 08536 1-833-Wegovy-1 Version: 2 WEGOVY® is a registered trademark of Novo Nordisk A/S. PATENT Information: http://novonordisk-us.com/products/product-patents.html © 2022 Novo Nordisk This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: August 2022 |


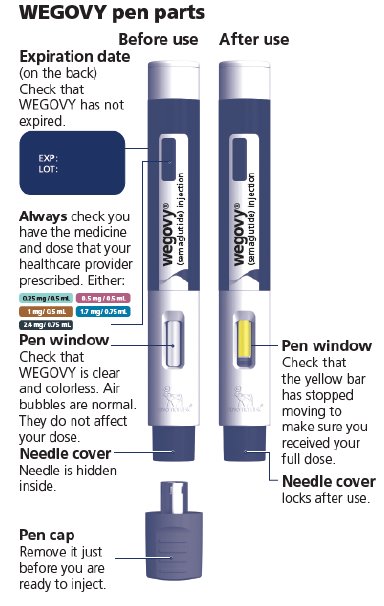
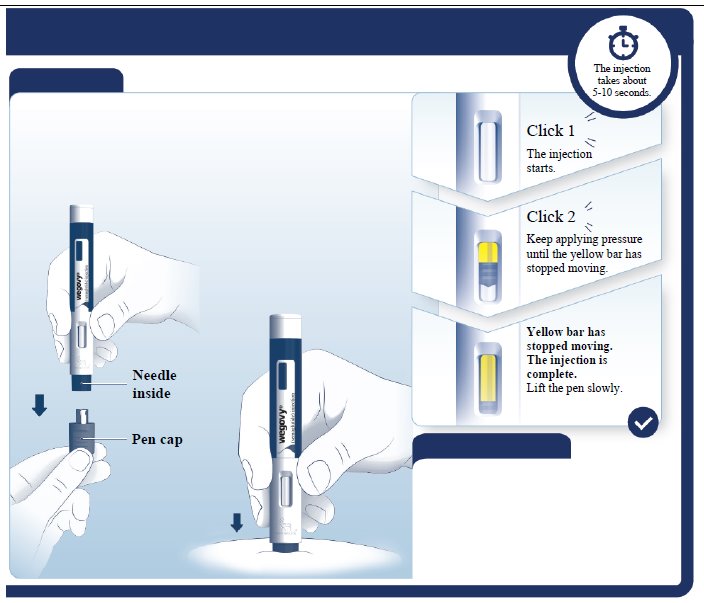

 How do I care for
my pen?
How do I care for
my pen?