PHOFAY Matte Sunscreen Liquid Foundation SPF 30, 1
PHOFAY Matte Sunscreen Liquid Foundation SPF 30, 1
18130858-429c-4ed4-a75b-5bc014481250
HUMAN OTC DRUG LABEL
Sep 4, 2025
Hangzhou Jingshen Trading Co., Ltd.
DUNS: 616067829
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ENSULIZOLE, HOMOSALATE, OCTINOXATE, TITANIUM DIOXIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (28)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package Labeling:
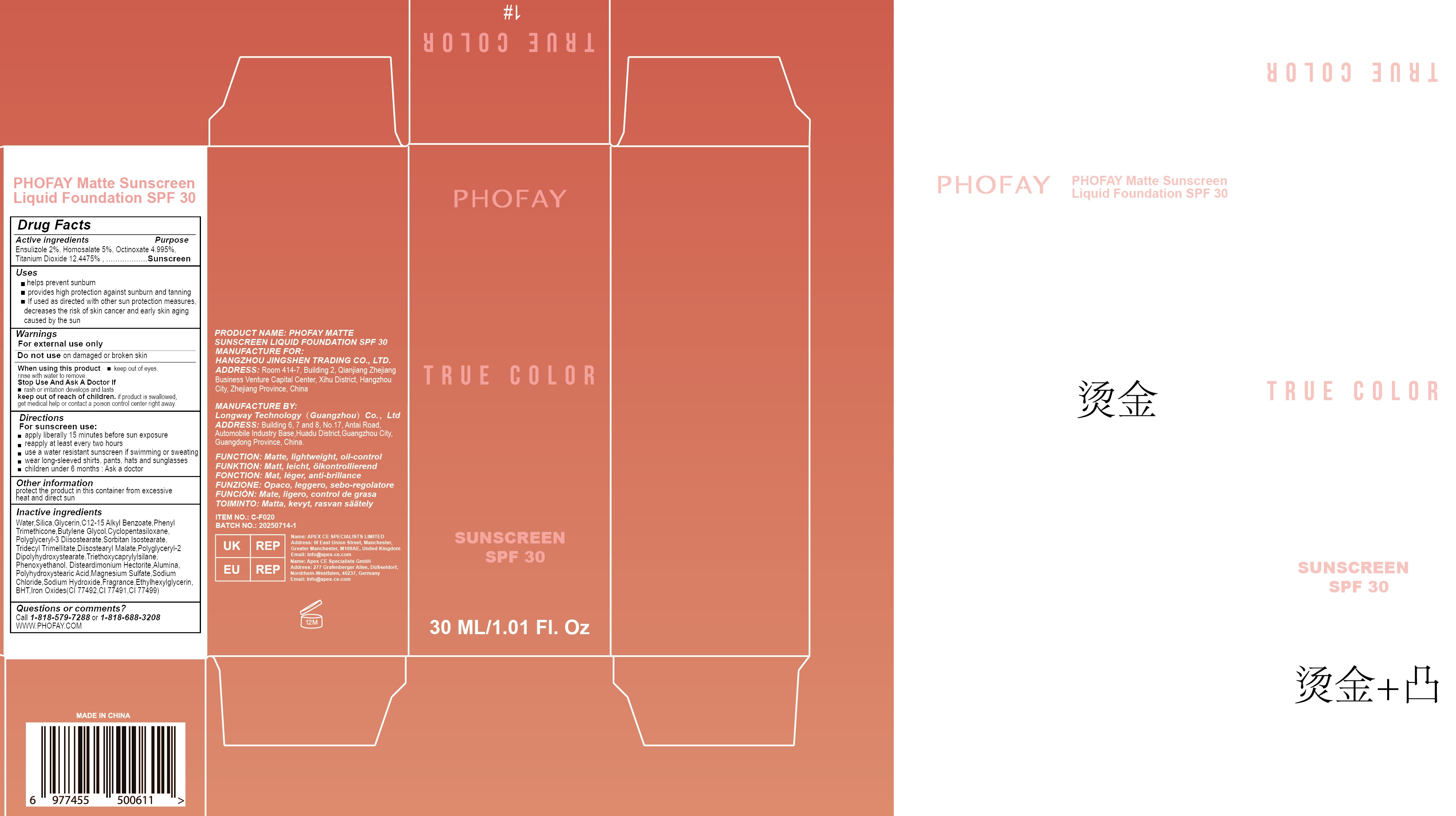

INDICATIONS & USAGE SECTION
Uses
- helps prevent sunburn
- provides high protection against sunburn and tanning
- lf used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun
WARNINGS SECTION
Warnings
For external use only
Do not use
on damaged or broken skin
When using this product
- keep out of eyes. rinse with water to remove.
Stop Use And Ask A Doctor lf
- rash or irritation develops and lasts
keep out of reach if children.
if product is swallowed, get medical help or contact a poison control center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months :Ask a doctor
SPL UNCLASSIFIED SECTION
Other information
protect the product in this container from excessive heat and direct sun
OTC - ACTIVE INGREDIENT SECTION
Active ingredients
Ensulizole 2%, Homosalate 5%, Octinoxate 4.995%, Titanium Dioxide 12.4475%
Purpose
Suscreen
INACTIVE INGREDIENT SECTION
Inactive ingredients
Water, Silica, Glycerin, C12-15 Alkyl Benzoate, Phenyl Trimethicone, Butylene Glycol, Cyclopentasiloxane, Polyglyceryl-3 Diisostearate, Sorbitan Isostearate, Tridecyl Trimellitate, Diisostearyl Malate, Polyglyceryl-2 Dipolyhydroxystearate, Triethoxycaprylylsilane, Phenoxyethanol, Disteardimonium Hectorite, Alumina, Polyhydroxystearic Acid, Magnesium Sulfate, Sodium Chloride, Sodium Hydroxide, Fragrance, Ethylhexylglycerin, BHT, lron Oxides (Cl77492, CI 77491, CI 77499)
OTC - QUESTIONS SECTION
Questions or comments?
Call1-818-579-7288or1-818-688-3208WWW.PHOFAY.COM
