Centratex
Centratex Capsules
4d4d823b-ff2f-4746-9bd7-44def2b81347
HUMAN PRESCRIPTION DRUG LABEL
Jan 31, 2024
Centurion Labs, LLC
DUNS: 016481957
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Iron, Sodium Ascorbate, Thiamine Mononitrate, Riboflavin, Pyridoxine Hydrochloride, Folic Acid, Cyanocobalamin, Niacinamide, Calcium Pantothenate, Zinc Sulfate, Magnesium Sulfate, Manganese Sulfate, and Cupric Sulfate Anhydrous
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (17)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Label
NDC 23359-100-10
Centratex
|
Iron (Ferrous Fumarate, anhydrous) |
106 mg |
|
Vitamin C (Sodium Ascorbate) |
200 mg |
|
Vitamin B1 (Thiamine Mononitrate) |
10 mg |
|
Vitamin B2 (Riboflavin) |
6 mg |
|
Vitamin B6 (Pyridoxine HCl) |
5 mg |
|
Vitamin B12 (Cyanocobalamin Concentrate) |
15 mcg |
|
Folic Acid |
1 mg |
|
Niacinamide (Nicotinamide) |
30 mg |
|
Pantothenic Acid (Calcium Pantothenate) |
10 mg |
|
Zinc (Zinc Sulfate) |
18.2 mg |
|
Magnesium (Magnesium Sulfate) |
6.9 mg |
|
Manganese (Manganese Sulfate) |
1.3 mg |
|
Copper (Copper Sulfate) |
0.8 mg |
CENTURION LABS
** Rx Only**
Net Contents: 100 capsules

INDICATIONS & USAGE SECTION
INDICATIONS
For the treatment of anemia due to lack of iron and low folate as in menorrhagia, pregnancy, puberty, excessive blood loss, and advanced age. Also for treatment of conditions where iron and vitamin C deficiency occur together, along with a poor intake or increased need for B-complex vitamins in chronic and acute illness, as well as cases of metabolic stress, and in periods of extended recovery.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
Centratex is contraindicated in patients with a known hypersensitivity to any of its ingredients. Iron compounds are contraindicated in patients with hemosiderosis, hemochromatosis, and hemolytic anemias. Folic acid may obscure signs and symptoms of pernicious anemia and is therefore a contraindication as well.
ADVERSE REACTIONS SECTION
ADVERSE REACTION
Allergic sensitizations have been reported following both oral and parenteral administration of folic acid. Gastrointestinal disturbances (anorexia, nausea, diarrhea, constipation) occur occasionally, but are usually mild and subside with continuation of therapy and physician encouragement. Although the absorption of iron is best when taken between meals, occasional G.I. disturbances may be controlled by giving Centratex shortly after meals.
WARNINGS SECTION
Warning
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B-12 is deficient.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Adults only: 1 capsule daily between meals, orally. Not recommended in children.
HOW SUPPLIED SECTION
HOW SUPPLIED
Centratex is supplied as a natural gelatin capsule with tan speckled powder. Centratex is available in bottles of 100 (NDC 23359-100-10) and bottles of 30 (NDC 23359-100-30) Dispense in a tight, light resistant container as defined in the USP/NF with a child resistant closure. Store at room temperature between 15-30 degrees C (59-86 degrees F) Keep in a cool, dry place.
PRECAUTIONS SECTION
PRECAUTIONS
General
Folic acid in doses above 0.1 mg – 0.4 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive.
PEDIATRIC USE
Safety and effectiveness in pediatric patients has not been established.
GERIATRIC USE
No clinical studies have been performed in patients over 65 to determine whether older persons respond differently from younger persons. Physicians should consider that elderly persons may have decreased hepatic, renal, or cardiac function.
SPL UNCLASSIFIED SECTION
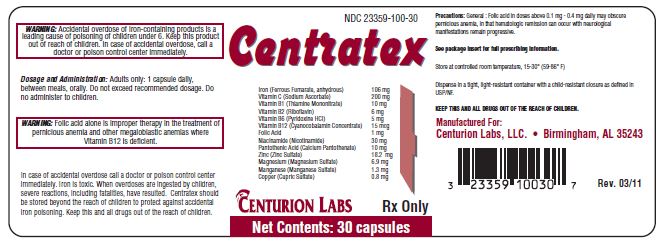
PRINCIPAL DISPLAY PANEL - 30 Capsule Bottle Lable
NDC 23359-100-30
Centratex
|
Iron (Ferrous Fumarate, anhydrous |
106 mg |
|
Vitamin C (Sodium Ascorbate) |
200 mg |
|
Vitamin B1 (Thiamine Mononitrate) |
10 mg |
|
Vitamin B2 (Riboflavin) |
6 mg |
|
Vitamin B6 (Pyridoxine HCl) |
5 mg |
|
Vitamin B12 (Cyanocobalamin Concentrate) |
15 mcg |
|
Folic Acid |
1 mg |
|
Niacinamide (Nicotinamide) |
30 mg |
|
Pantothenic Acid (Calcium Pantothenate) |
10 mg |
|
Zinc (Zinc Sulfate) |
18.2 mg |
|
Magnesium (Magnesium Sulfate) |
6.9 mg |
|
Manganese (Manganese Sulfate) |
1.3 mg |
|
Copper (Cupric Sulfate) |
0.8 mg |
CENTURION LABS Rx Only
Net Contents: 30 capsules
DESCRIPTION SECTION
DESCRIPTION
Active Ingredients (Each capsule)
|
Ferrous Fumerate |
106 mg(Iron) |
|
Vitamin C (Sodium Ascorbate) |
200 mg |
|
Vitamin B1 (Thiamine Mononitrate) |
10 mg |
|
Vitamin B2 (Riboflavin) |
6 mg |
|
Vitamin B6 (Pyridoxine HCL) |
5 mg |
|
Folic Acid |
1 mg |
|
Vitamin B12 |
15 mcg |
|
Niacinamide |
30 mg |
|
Pantothenic Acid |
10 mg |
|
Zinc (Zinc Sulfate) |
18.2 mg |
|
Magnesium (Magnesium Sulfate) |
6.9 mg |
|
Copper (Copper Sulfate) |
0.8 mg |
|
Manganese (Manganese Sulfate) |
1.3 mg |
Inactive Ingredients
Microcrystalline Cellulose, Silica, Magnesium Stearate, Gelatin Capsule
