Prenatal Plus Vitamins
Prenatal Vitamins Plus Low Iron
1b8ef3ea-5563-48ef-ba5e-27e0b454efb9
HUMAN PRESCRIPTION DRUG LABEL
Sep 30, 2025
REMEDYREPACK INC.
DUNS: 829572556
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
prenatal with ferrous fumarate and folic acid
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (25)
Drug Labeling Information
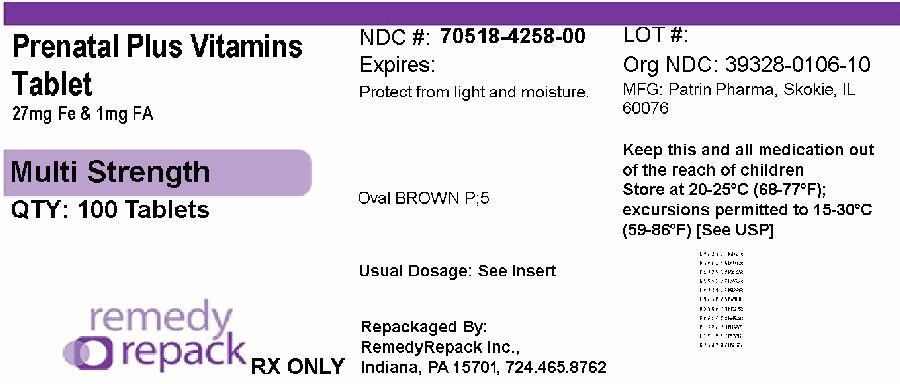
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
DRUG: Prenatal Plus Vitamins
GENERIC: prenatal with ferrous fumarate and folic acid
DOSAGE: TABLET
ADMINSTRATION: ORAL
NDC: 70518-4258-0
COLOR: brown
SHAPE: OVAL
SCORE: No score
SIZE: 9 mm
IMPRINT: P;5
PACKAGING: 100 in 1 BOTTLE, PLASTIC
ACTIVE INGREDIENT(S):
- Vitamin A Acetate 924ug in 1
- Beta Carotene 276ug in 1
- Ascorbic Acid 120mg in 1
- Cholecalciferol 10ug in 1
- .Alpha.-TocopheroL Acetate, DL- 10mg in 1
- Thiamine Mononitrate 1.84mg in 1
- Riboflavin 3mg in 1
- Niacinamide 20mg in 1
- Pyridoxine Hydrochloride 10mg in 1
- Folic Acid 1667ug in 1
- Cyanocobalamin 12ug in 1
- Calcium Carbonate 200mg in 1
- Ferrous Fumarate 27mg in 1
- Zinc Oxide 25mg in 1
- Cupric Oxide 2mg in 1
INACTIVE INGREDIENT(S):
- Hypromellose, Unspecified
- Magnesium Stearate
- Maltodextrin
- Microcrystalline Cellulose
- Polyethylene Glycol 400
- Stearic Acid
- Titanium Dioxide
- FD&C Blue NO. 2
- FD&C Red NO. 40
- FD&C Yellow NO. 6
BOXED WARNING SECTION
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under six. Keep this product out of the reach of children. In case of accidental overdose, call a Physician or Poison Control Center immediately.
DESCRIPTION SECTION
Description
Prenatal Plus Vitamins is a prescription folic acid containing dietary vitamin and mineral supplement for women before, during and after pregnancy.
DOSAGE & ADMINISTRATION SECTION
Directions
Use before, during and after pregnancy under the supervision of a medical professional. One tablet daily or as prescribed.
PRECAUTIONS SECTION
Caution
Folic acid may partially correct the hematological damage due to Vitamin B-12 deficiency of pernicious anemia while the associated neurological damage progresses.
STORAGE AND HANDLING SECTION
Storage
Store at room temperature, USP.**Protect from light and moisture.**Dispense in a tight, light-resistant container. Contact with moisture may discolor or erode tablets.
SPL UNCLASSIFIED SECTION
Repackaged and Distributed By:
Remedy Repack, Inc.
625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
