Pain Relief
73557-175
3ed51136-ea8e-8101-e063-6394a90acd4c
HUMAN OTC DRUG LABEL
Sep 22, 2025
Shanghai Chuangshi Medical Technology (Group) Co., Ltd.
DUNS: 546872672
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lidocaine and Menthol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package label. Principal display panel
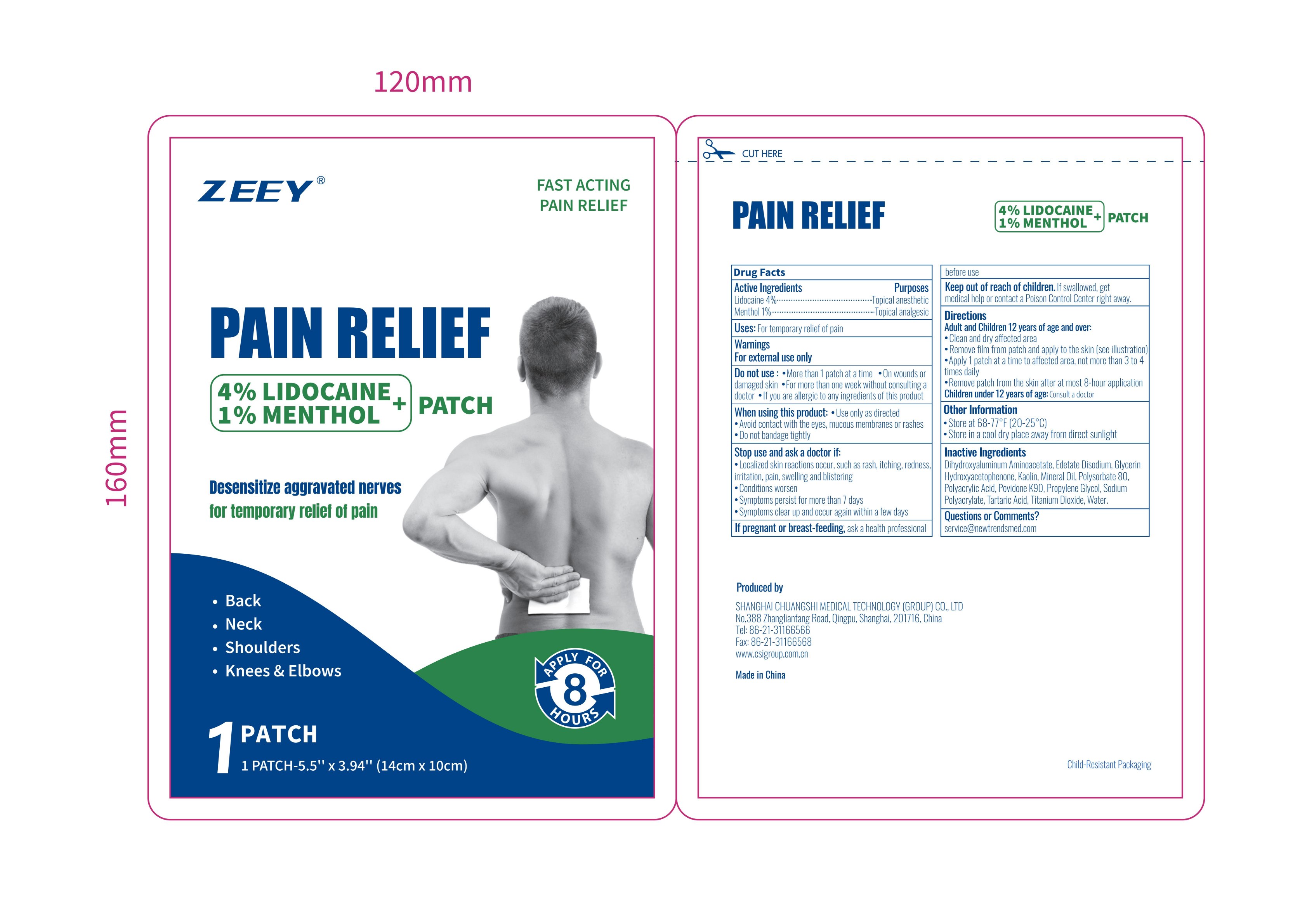
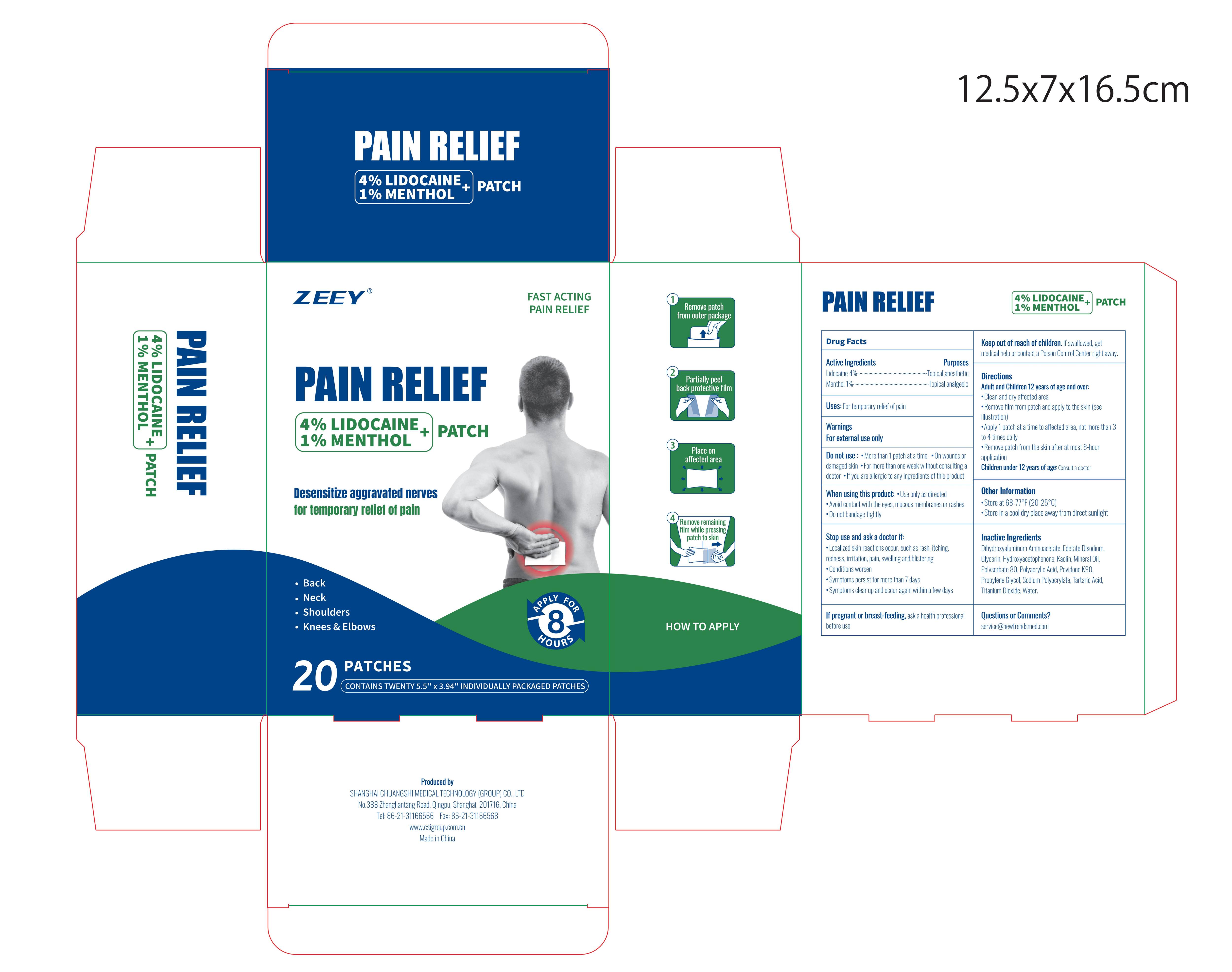
INDICATIONS & USAGE SECTION
Uses
For temporary relief of pain
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Lidocaine 4% w/w ...... Purpose: Topical anesthetic
Menthol 1% w/w ...... Purpose: Topical analgesic
OTC - PURPOSE SECTION
Purposes
Topical anesthetic (Lidocaine)
Topical analgesic (Menthol)
WARNINGS SECTION
Warnings
For external use only
OTC - WHEN USING SECTION
When using this product:
- Use only as directed
- Avoid contact with the eyes, mucous membranes or rashes
- Do not bandage tightly
OTC - DO NOT USE SECTION
Do not use:
- More than 1 patch at a time
- On wounds or damaged skin
- For more than one week without consulting a doctor
- If you are allergic to any ingredients of this product
OTC - STOP USE SECTION
Stop use and ask a doctor if:
- Localized skin reactions occur, such as rash, itching, redness, irritation, pain, swelling and blistering
- Condition worsen
- Symptoms persist for more than 7 days
- Symptoms clear up and occur again within a few days
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding,
Ask a health professional before use
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away
DOSAGE & ADMINISTRATION SECTION
Directions
Adult and Children 12 years of age and over:
- Clean and dry affected area
- Remove film from patch and apply to the skin (see illustration)
- Apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- Remove patch from the skin after at most 8-hour application
**Children under 12 years of age:**Consult a doctor
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Dihydroxyaluminium Aminoacetate, Edetate Disodium,Glycerin, Hydroxyacetophenone,Kaolin, Mineral Oil,Polysorbate 80, Polyacrylic Acid, Povidone K90, Propylene Glycol,Sodium Polyacrylate,Tartaric Acid,Titanium Dioxide,Water
OTC - QUESTIONS SECTION
Questions or Comments
service@newtrendsmed.com
STORAGE AND HANDLING SECTION
Other information
- Store at 68-77℉ (20-25℃)
- Store in a cool dry place away from direct sunlight
