Fiasp
These highlights do not include all the information needed to use FIASP safely and effectively. See full prescribing information for FIASP.FIASP (insulin aspart) injection, for subcutaneous or intravenous useInitial U.S. Approval: 2000
834e7efc-393f-4c55-9125-628562a8a5cf
HUMAN PRESCRIPTION DRUG LABEL
Jun 21, 2023
Novo Nordisk
DUNS: 622920320
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
insulin aspart injection
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
insulin aspart injection
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
insulin aspart injection
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
insulin aspart
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
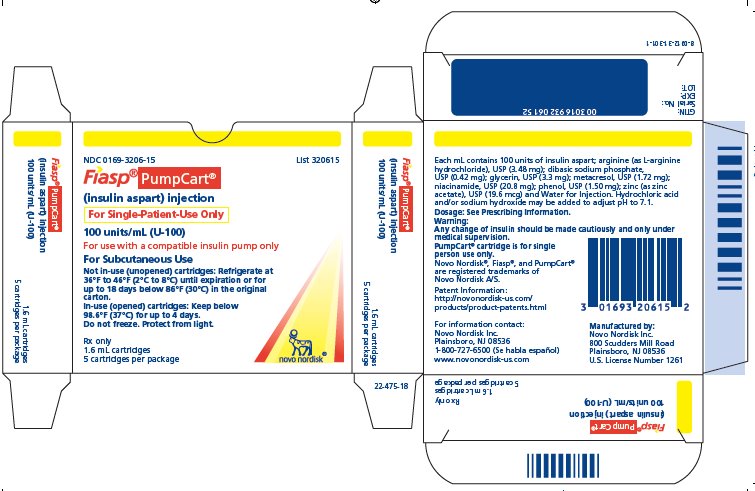
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel - PumpCart
NDC 0169-3206-15 List 320615
**Fiasp****®**PumpCart®
(insulin aspart) injection
For Single-Patient Use Only
100 units/mL (U-100)
For use with a compatible insulin pump only
For Subcutaneous Use
Not in-use (unopened) cartridges: Refrigerate at 36°F to 46°F (2°C to 8°C) until expiration or for up to 18 days below 86°F (30°C) in the original carton.
In-use (opened) cartridges: Keep below 98.6°F (37°C) for up to 4 days.
Do not freeze. Protect from light.
Rx only
1.6 mL cartridges
5 cartridges per package
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Never Share a FIASP FlexTouch Pen, PenFill Cartridge or PenFill
Cartridge Device Between Patients
FIASP FlexTouch disposable pen, PenFill cartridge and PenFill cartridge devices should never be shared between patients, even if the needle is changed. Patients using FIASP vials should never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
5.2 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia [see Warnings and Precautions (5.3)] or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia [see Adverse Reactions (6.1, 6.2)].
Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. For patients with type 2 diabetes mellitus, dosage adjustments in concomitant anti-diabetic treatment may be needed.
5.3 Hypoglycemia
Hypoglycemia is the most common adverse reaction of all insulin therapies, including FIASP [see Adverse Reactions (6.1)]. Severe hypoglycemia can cause seizures, may lead to unconsciousness, may be life-threatening, or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). FIASP, or any insulin, should not be used during episodes of hypoglycemia [see Contraindications (4)].
Hypoglycemia can happen suddenly and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers) [see Drug Interactions (7)], or in patients who experience recurrent hypoglycemia.
Risk Factors for Hypoglycemia
The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. The timing of hypoglycemia usually reflects the time-action profile of the administered insulin formulation. As with all insulin preparations, the glucose lowering effect time course of FIASP may vary in different individuals or at different times in the same individual and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature [see Use in Specific Populations (8.4), Clinical Pharmacology (12.2)].
Other factors which may increase the risk of hypoglycemia include changes in meal pattern (e.g., macronutrient content or timing of meals), changes in level of physical activity, or changes to co-administered medication [seeDrug Interactions (7)]. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [seeUse in Specific Populations (8.6, 8.7)].
Risk Mitigation Strategies for Hypoglycemia
Patients and caregivers must be educated to recognize and manage hypoglycemia. Self-monitoring of blood glucose plays an essential role in the prevention and management of hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended.
5.4 Hypoglycemia Due to Medication Errors
Accidental mix-ups between insulin products have been reported. To avoid medication errors between FIASP and other insulins, instruct patients to always check the insulin label before each injection.
5.5 Hypokalemia
All insulin products, including FIASP, can cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to potassium concentrations).
5.6 Hypersensitivity and Allergic Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including FIASP [see Adverse Reactions (6.1)]. If hypersensitivity reactions occur, discontinue FIASP; treat per standard of care and monitor until symptoms and signs resolve. FIASP is contraindicated in patients who have had hypersensitivity reactions to insulin aspart, or any of the excipients in FIASP [see Contraindications (4)].
5.7 Fluid Retention and Heart Failure with Concomitant Use of PPAR-Gamma
Agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including FIASP, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR- gamma agonist must be considered.
5.8 Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction
Pump or infusion set malfunctions can lead to a rapid onset of hyperglycemia and ketoacidosis. Prompt identification and correction of the cause of hyperglycemia or ketosis is necessary. Interim therapy with subcutaneous injection of FIASP may be required. Patients using continuous subcutaneous insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure [see Dosage and Administration (2.2), How Supplied/Storage and Handling (16.2), and Patient Counseling Information (17)].
•
Never share a FIASP FlexTouch pen, PenFill cartridge or PenFill cartridge device between patients, even if the needle is changed (5.1).
•
Hyperglycemia or hypoglycemia with changes in insulin regimen: Make changes to a patient’s insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) under close medical supervision with increased frequency of blood glucose monitoring (5.2).
•
Hypoglycemia: May be life-threatening. Increase frequency of glucose monitoring with changes to: insulin dosage, co-administered glucose lowering medications, meal pattern, physical activity; and in patients with renal impairment or hepatic impairment or hypoglycemia unawareness (5.3).
•
Hypoglycemia due to medication errors: Accidental mix-ups between insulin products can occur. Instruct patients to check insulin labels before injection (5.4).
•
Hypokalemia: May be life-threatening. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated (5.5).
•
Hypersensitivity reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue FIASP, monitor and treat if indicated (5.6).
•
Fluid retention and heart failure with concomitant use of thiazolidinediones (TZDs): Observe for signs and symptoms of heart failure; consider dosage reduction or discontinuation if heart failure occurs (5.7).
•
Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction: Monitor glucose and administer FIASP by subcutaneous injection if pump malfunction occurs (5.8).
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Table 5 includes clinically significant drug interactions with FIASP.
Table 5. Clinically Significant Drug Interactions with FIASP
|
Drugs That May Increase the Risk of Hypoglycemia | |
|
Drugs: |
Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics. |
|
Intervention: |
Dose reductions and increased frequency of glucose monitoring may be required when FIASP is co-administered with these drugs. |
|
Drugs That May Decrease the Blood Glucose Lowering Effect of FIASP | |
|
Drugs: |
Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. |
|
Intervention: |
Dose increases and increased frequency of glucose monitoring may be required when FIASP is co-administered with these drugs. |
|
Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of FIASP | |
|
Drugs: |
Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. |
|
Intervention: |
Dose adjustment and increased frequency of glucose monitoring may be required when FIASP is co-administered with these drugs. |
|
Drugs That May Blunt Signs and Symptoms of Hypoglycemia | |
|
Drugs: |
Beta-blockers, clonidine, guanethidine, and reserpine. |
|
Intervention: |
Increased frequency of glucose monitoring may be required when FIASP is co- administered with these drugs. |
•
Drugs that Increase Hypoglycemia Risk or Increase or Decrease Blood Glucose Lowering Effect: Adjustment of dosage may be needed; closely monitor blood glucose (7).
•
Drugs that Blunt Hypoglycemia Signs and Symptoms (e.g., beta-blockers, clonidine, guanethidine, and reserpine): Increased frequency of glucose monitoring may be required (7).
OVERDOSAGE SECTION
10 OVERDOSAGE
Excess insulin administration may cause hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.5)]. Mild episodes of hypoglycemia usually can be treated with oral glucose. Adjustments in drug dosage, meal patterns, or exercise, may be needed. More severe episodes with coma, seizure or neurologic impairment may be treated with an intramuscular/subcutaneous glucagon product for emergency use or concentrated intravenous glucose. Sustained carbohydrate intake and observation may be necessary because hypoglycemia may recur after apparent clinical recovery. Hypokalemia must be corrected appropriately.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In 52-week studies, Sprague-Dawley rats were dosed subcutaneously with insulin aspart at 10, 50, and 200 units/kg/day (approximately 2, 8, and 32 times the human subcutaneous dose of 1.0 units/kg/day, based on units/body surface area, respectively). At a dose of 200 units/kg/day, insulin aspart increased the incidence of mammary gland tumors in females when compared to untreated controls. The incidence of mammary tumors for insulin aspart was not significantly different than for regular human insulin. The relevance of these findings to humans is not known.
Insulin aspart was not genotoxic in the following tests: Ames test, mouse lymphoma cell forward gene mutation test, human peripheral blood lymphocyte chromosome aberration test, in vivo micronucleus test in mice, and in ex vivo UDS test in rat liver hepatocytes.
In fertility studies in male and female rats, at subcutaneous doses up to 200 units/kg/day (approximately 32 times the human subcutaneous dose, based on units/body surface area), no direct adverse effects on male and female fertility, or general reproductive performance of animals was observed.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-Approved Patient Labeling (Patient Information and Instructions for Use).
Never Share a FIASP FlexTouch Pen Device, PenFill Cartridge or PenFill Cartridge Device Between Patients
Advise patients that they should never share a FIASP FlexTouch pen device, PenFill cartridge or PenFill cartridge devices with another person, even if the needle is changed, because doing so carries a risk for transmission of blood-borne pathogens. Advise patients using FIASP vials not to share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens [see Warnings and Precautions (5.1)].
Hyperglycemia or Hypoglycemia
Inform patients that hypoglycemia is the most common adverse reaction with insulin. Instruct patients on self-management procedures including glucose monitoring, proper injection technique, and management of hypoglycemia and hyperglycemia, especially at initiation of FIASP therapy. Instruct patients on handling of special situations such as intercurrent conditions (illness, stress, or emotional disturbances), an inadequate or skipped insulin dose, inadvertent administration of an increased insulin dose, inadequate food intake, and skipped meals. Instruct patients on the management of hypoglycemia [see Warnings and Precautions (5.3)].
Inform patients that their ability to concentrate and react may be impaired as a result of hypoglycemia. Advise patients who have frequent hypoglycemia or reduced or absent warning signs of hypoglycemia to use caution when driving or operating machinery.
Advise patients that changes in insulin regimen can predispose to hyperglycemia or hypoglycemia and that changes in insulin regimen should be made under close medical supervision [see Warnings and Precautions (5.2)].
Hypersensitivity Reactions
Advise patients that hypersensitivity reactions have occurred with FIASP. Inform patients on the symptoms of hypersensitivity reactions [see Warnings and Precautions (5.6)].
Hypoglycemia due to Medication Errors
Instruct patients to always check the insulin label before each injection to avoid mix-ups between insulin products.
Patients Using Continuous Subcutaneous Insulin Pumps
•
Train patients in intensive insulin therapy with multiple injections and in the function of their pump and pump accessories.
•
Instruct patients to follow healthcare provider recommendations when setting basal and meal time infusion rate.
•
Refer to the continuous subcutaneous infusion pump user manual to see if FIASP can be used with the pump. See recommended reservoir and infusion sets in the insulin pump user manual.
•
Instruct patients to change FIASP in the pump reservoir at least every 6 days, or replace the PumpCart cartridge at least every 4 days or according to the pump user manual, whichever is shorter; infusion sets and infusion set insertion sites should be changed in accordance with the manufacturers’ user manual. By following this schedule, patients avoid insulin degradation, infusion set occlusion, and loss of the insulin preservative.
•
Instruct patients to discard insulin exposed to temperatures higher than 37°C (98.6°F).
•
Instruct patients to inform physician and select a new site for infusion if infusion site becomes erythematous, pruritic, or thickened.
•
Instruct patients on the risk of rapid hyperglycemia and ketosis due to pump malfunction, infusion set occlusion, leakage, disconnection or kinking, and degraded insulin. Instruct patients on the risk of hypoglycemia from pump malfunction. If these problems cannot be promptly corrected, instruct patients to resume therapy with subcutaneous insulin injection and contact their physician [see Warnings and Precautions (5) and How Supplied/Storage and Handling (16.2)].
Version: 8
Novo Nordisk®, FIASP®, NovoLog®, FlexTouch®, PenFill® and PumpCart® are registered trademarks of Novo Nordisk A/S.
PATENT Information: http://novonordisk-us.com/products/product-patents.html
© 2023
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
U.S. License 1261
For information about FIASP contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
1-800-727-6500
www.novonordisk-us.com
SPL PATIENT PACKAGE INSERT SECTION
Patient Information
FIASP**®**** (fee’ asp)**
(insulin aspart)
Injection, for subcutaneous or intravenous use
Do not share your FIASP with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
What is FIASP?
•
FIASP is a man-made insulin that is used to control high blood sugar in adults and children with diabetes mellitus.
Who should not take FIASP?
Do not take FIASP if you:
•
are having an episode of low blood sugar (hypoglycemia).
•
have an allergy to insulin aspart or any of the ingredients in FIASP.
Before taking FIASP, tell your healthcare provider about all your medical conditions including, if you:
•
have kidney problems.
•
have liver problems.
•
are pregnant or plan to become pregnant. Talk with your healthcare provider about the best way to control your blood sugar if you plan to become pregnant or while you are pregnant.
•
are breastfeeding or plan to breastfeed. It is not known if FIASP passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby while using FIASP.
•
are taking new prescription or over-the-counter medicines, vitamins, or herbal supplements.
Before you start taking FIASP, talk to your healthcare provider about low blood sugar and how to manage it.
How should I take FIASP?
•
**Read the Instructions for Use** that come with your FIASP.
•
Take FIASP exactly as your healthcare provider tells you to.
•
**FIASP starts acting fast.** You should take your dose of FIASP at the beginning of the meal or within 20 minutes after starting a meal.
•
Know the type and strength of insulin you take.**Do not**change the type of insulin you take unless your healthcare provider tells you to. The amount of insulin and the best time for you to take your insulin may need to change if you take different types of insulin.
•
If you miss a dose of FIASP, monitor your blood sugar levels to decide if an insulin dose is needed. Continue with your regular dosing schedule at the next meal.
•
**Check your blood sugar levels.** Ask your healthcare provider what your blood sugars should be and when you should check your blood sugar levels.
•
**Do not reuse or share needles with other people. You may give other people a serious infection or get a serious infection from them.**
•
FIASP can be injected under the skin (subcutaneously) of your stomach area, upper legs, or upper arms, or by continuous infusion under the skin (subcutaneously) through an insulin pump into an area of your body recommended in the instructions that come with your insulin pump.
•
**Change (rotate) your injection sites within the area you choose with each dose**to reduce your risk of getting pits in skin or thickened skin (lipodystrophy) and skin with lumps (localized cutaneous amyloidosis) at the injection sites.
o
**Do not** use the exact same spot for each injection.
o
**Do not** inject where the skin has pits, is thickened, or has lumps.
o
**Do not** inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
What should I avoid while taking FIASP?
While taking FIASP do not:
•
Drive or operate heavy machinery until you know how FIASP affects you.
•
Drink alcohol or use prescription or over-the-counter medicines that contain alcohol.
What are the possible side effects of FIASP?
FIASP may cause serious side effects that can lead to death, including:
•
**low blood sugar (hypoglycemia).**Signs and symptoms that may indicate low blood sugar include:
|
o |
o |
o |
|
o |
o |
o |
|
o |
o |
o |
|
o |
•
**low potassium in your blood (hypokalemia).**
•
**serious allergic reactions (whole body reactions).** Get emergency medical help right away, if you have any of these signs or symptoms of a severe allergic reaction:
o
a rash over your whole body, trouble breathing, a fast heartbeat, swelling of your face, tongue or throat, sweating, extreme drowsiness, dizziness, confusion.
•
**heart failure.**Taking certain diabetes pills called TZDs (thiazolidinediones) with FIASP may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with FIASP. Your healthcare provider should monitor you closely while you are taking TZDs with FIASP. Tell your healthcare provider if you have any new or worse symptoms of heart failure including shortness of breath, swelling of your ankles or feet, sudden weight gain. Treatment with TZDs and FIASP may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
Your insulin dose may need to change because of:
|
• |
• |
|
• |
• |
|
• |
Common side effects of FIASP may include:
•
skin problems such as eczema, rash, itching, redness and swelling of your skin (dermatitis)
•
reactions at the injection site such as itching, rash
•
skin thickening or pits at the injection site (lipodystrophy)
•
weight gain
These are not all the possible side effects of FIASP. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of FIASP.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about FIASP that is written for health professionals. Do not use FIASP for a condition for which it was not prescribed. Do not give FIASP to other people, even if they have the same symptoms that you have. It may harm them.
What are the ingredients in FIASP?
Active Ingredient: insulin aspart
**Inactive Ingredients:**arginine, dibasic sodium phosphate, glycerin, metacresol, niacinamide, phenol, zinc and water for injection.
Manufactured by:
Novo Nordisk Inc.
U.S. License Number 1261
For more information, go to www.novonordisk-us.com or call 1-800-727-6500.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 06/2023
INSTRUCTIONS FOR USE
FIASP (fee’ asp)
(insulin aspart) injection
10 mL multiple-dose vial (100 units/mL, U-100)
Read this Instructions for Use before you start taking FIASP and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. The vial is not recommended for use by the blind or visually impaired without the assistance of a person trained in the proper use of the product and insulin syringe.
Do not reuse or share syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
Supplies you will need to give your FIASP injection:
•
a 10 mL FIASP vial
•
a U-100 insulin syringe and needle
•
2 alcohol swabs
•
1 sharps container for throwing away used needles and syringes. See “Disposing of your used needles and syringes” at the end of these instructions.

Preparing your FIASP dose:
•
**Do not** roll or shake the FIASP vial. Shaking the FIASP vial right before the dose is drawn into the syringe may cause bubbles or foam. This can cause you to draw up the wrong dose of insulin.
•
The tamper-resistant cap should not be loose or damaged before the first use.**Do not** use if the tamper-resistant cap is loose or damaged before using FIASP for the first time.
•
Wash your hands with soap and water.
•
**Before you start to prepare your injection, check the FIASP label to make sure that you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.**
•
Check that the FIASP vial is not cracked or damaged.**Do not** use if the FIASP vial is cracked or damaged.
•
FIASP should look clear and colorless.**Do not** use FIASP if it is thick, cloudy, or is colored.
•
**Do not** use FIASP past the expiration date printed on the label.
|
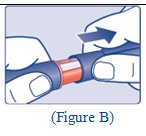
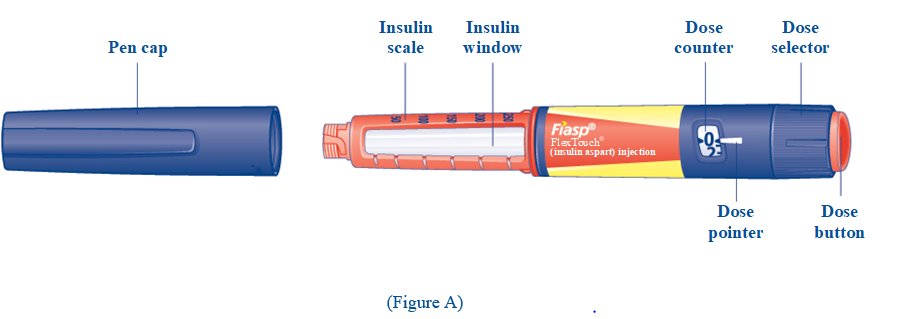
**Step 1:**Pull off the tamper-resistant cap (See Figure A). **Step 2:**Wipe the rubber stopper with an alcohol swab (See Figure B). |
(Figure A Figure B) |
|
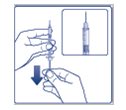
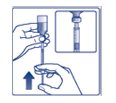
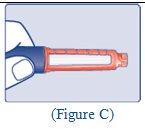
Step 3: Hold the syringe with the needle pointing up. Pull down on the plunger until the tip of the plunger reaches the line for the number of units for your prescribed dose (See Figure C). |
(Figure C) |
|
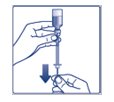
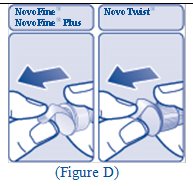
**Step 4:**Push the needle through the rubber stopper of the FIASP vial (See Figure D). |
(Figure D) |
|
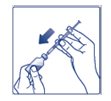
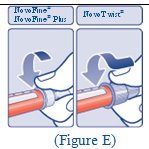
**Step 5:**Push the plunger all the way in. This puts air into the FIASP vial (See Figure E). |
(Figure E) |
|
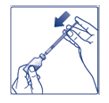
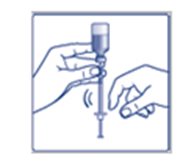
**Step 6:**Turn the FIASP vial and syringe upside down and slowly pull the plunger down until the tip of the plunger is a few units past the line for your dose (See Figure F). If there are air bubbles, tap the syringe gently a few times to let any air bubbles rise to the top (See Figure G). |
(Figure F)
(Figure G) |
|
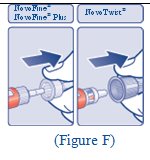
**Step 7:**Slowly push the plunger up until the tip of the plunger reaches the line for your prescribed FIASP dose (See Figure H). |
(Figure H) |
|
**Step 8:**Check the syringe to make sure you have the right dose of FIASP. | |
|
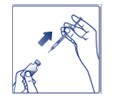
**Step 9:**Pull the syringe out of the rubber stopper on the vial (See Figure I). |
(Figure I) |
Giving your FIASP injection:
•
Inject your FIASP exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
•
You should take your dose of FIASP at the start of a meal or within 20 minutes after starting a meal.
•
FIASP can be injected under the skin (subcutaneously) of your stomach area, upper legs, or upper arms, infused in an insulin pump into an area of your body recommended in the instructions that come with your insulin pump, or given through a needle in your arm (intravenously) by your healthcare provider.**Do not** inject FIASP into your muscle.
•
If you use FIASP in an insulin pump, you should change the infusion sets and the infusion set insertion site according to the pump manufacturers’ user manual. The insulin in the reservoir should be changed at least every 6 days even if you have not used all of the insulin.
•
If you use FIASP in an insulin pump, see your insulin pump manual for instructions or talk to your healthcare provider. Your healthcare provider should provide recommendations for appropriate basal and meal time infusion rates.
•
Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.**Do not** use the same injection site for each injection.**Do not** inject where the skin has pits, is thickened, or has lumps.**Do not** inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
•
**Do not** dilute or mix FIASP with any other type of insulin products or solutions.
|
Step 10: Choose your injection site (thighs, upper arms, or abdomen) and wipe the skin with an alcohol swab (See Figure J). Let the injection site dry before you inject your dose. |
(Figure J) |
|
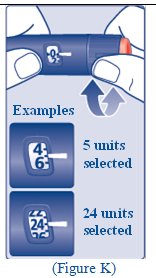
**Step 11:**Insert the needle into your skin. Push down on the plunger to inject your dose (See Figure K). Make sure you have injected all the insulin in the syringe. |
(Figure K) |
|
Step 12: Pull the needle out of your skin. After your injection you may see a drop of FIASP at the needle tip. This is normal and does not affect the dose you just received (See Figure L). • |
(Figure L) |
After your injection:
•
**Do not** recap the needle. Recapping the needle can lead to needle stick injury.
Disposing of your used needles and syringes:
Put your used insulin needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
o
made of a heavy-duty plastic;
o
can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out;
o
upright and stable during use;
o
leak-resistant, and
o
properly labeled to warn of hazardous waste inside the container.
•
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share needles or syringes with another person. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: [**http://www.fda.gov/safesharpsdisposal.**](http://www.fda.gov/safesharpsdisposal.)
•
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store FIASP?
•
**Do not** freeze FIASP.**Do not** use FIASP if it has been frozen.
•
Keep FIASP away from excessive heat or light.
All unopened vials:
•
Store unopened FIASP vials in the refrigerator at 36°F to 46°F (2°C to 8°C) or at room temperature below 86°F (30°C).
•
If unopened vials have been stored in the refrigerator, vials may be used until the expiration date printed on the label.
•
If unopened vials have been stored at room temperature, vials should be thrown away after 28 days.
After vials have been opened:
•
Opened FIASP vials can be stored in the refrigerator at 36°F to 46°F (2°C to 8°C) or at room temperature below 86°F (30°C).
•
Throw away all opened FIASP vials after 28 days (including 6 days pump in-use time), even if they still have insulin left in them.
General information about the safe and effective use of FIASP
•
Always use a new syringe and needle for each injection to help ensure sterility and prevent blocked needles.
•
**Do not** reuse or share syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
•
Keep FIASP vials, syringes, and needles out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-800-727-6500
U.S. License No. 1261
FIASP® is a registered trademark of Novo Nordisk A/S.
PATENT Information: http://novonordisk-us.com/products/product-patents.html
© 2023 Novo Nordisk
For information about FIASP® contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
1-800-727-6500
Revised: 06/2023
INSTRUCTIONS FOR USE
FIASP**®**** [fee’ asp]FlexTouch®**
(insulin aspart)
injection, for subcutaneous use
3 mL single-patient-use pen: 100 units/mL (U-100)
•
**Do not** share your FIASP FlexTouch Pen with other people, even if the needle is changed. You may give other people a serious infection, or get a serious infection from them.
•
**FIASP FlexTouch Pen (“Pen”) is a prefilled disposable, single-patient-use pen** containing 300 units of U-100 FIASP (insulin aspart) injection. You can inject from 1 to 80 units in a single injection. The units can be increased by 1 unit at a time.
•
People who are blind or have vision problems should not use the Pen without help from a person trained to use the Pen.
•
**Do not** use a syringe to remove FIASP from the FlexTouch Pen.
Supplies you will need to give your FIASP injection:
•
FIASP FlexTouch Pen
•
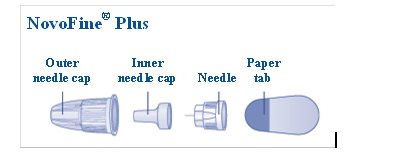
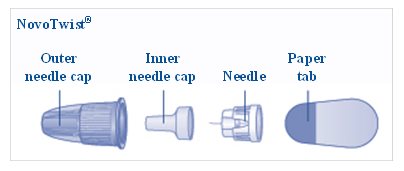
a new NovoFine, NovoFine Plus or NovoTwist needle
•
alcohol swab
•
a sharps container for throwing away used Pens and needles.**See “After your injection” at the end of these instructions.**
Preparing your FIASP FlexTouch Pen:
•
Wash your hands with soap and water.
•
Before you start to prepare your injection, check the FIASP FlexTouch Pen label to make sure you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin.
•
FIASP should look clear and colorless.**Do not** use FIASP if it is thick, cloudy, or is colored.
•
**Do not** use FIASP past the expiration date printed on the label or 28 days after you start using the Pen.
•
**Always**use a new needle for each injection to help ensure sterility and prevent blocked needles.**Do not**reuse or share needles with another person. You may give other people a serious infection, or get a serious infection from them.
|
Step 1: • |
|
|
Step 2: • |
|
|
Step 3: • • |
|
|
Step 4: • |
|
|
Step 5: • |
|
|
Step 6: • |
|
Priming your FIASP FlexTouch Pen:
|
Step 7: • |
|
|
Step 8: • |
|
|
Step 9: • • o o |
|
Selecting your dose:
|
Step 10: Check to make sure the dose selector is set at 0. • o o o |
|
|
• |
|
|
• o o |
Giving your injection:
•
Inject your FIASP exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
•
You should take your dose of FIASP at the start of a meal or within 20 minutes after starting a meal.
•
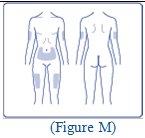
FIASP can be injected under the skin (subcutaneously) of your stomach area (abdomen), upper legs (thighs) or upper arms.**Do not** inject FIASP into your muscle.
•
Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.**Do not** use the same injection site for each injection.**Do not** inject where the skin has pits, is thickened, or has lumps.**Do not** inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
|
Step 11: • |
|
|
Step 12: • o |
|
|
Step 13: • o • o o o |
|
|
Step 14: • o |
|
|
Step 15: • o • o |
|
|
Step 16: • |
|
After your injection:
•
Put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
•
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
o
made of a heavy-duty plastic
o
can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
o
upright and stable during use
o
leak-resistant
o
properly labeled to warn of hazardous waste inside the container
•
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share needles or syringes with another person. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: <http://www.fda.gov/safesharpsdisposal>.
•
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store my FIASP FlexTouch Pen?
Before use:
•
Store unused FIASP FlexTouch Pens in the refrigerator at 36°F to 46°F (2°C to 8°C) or at room temperature below 86°F (30°C).
•
**Do not** freeze FIASP.**Do not** use FIASP if it has been frozen.
•
Unused Pens may be used until the expiration date printed on the label, if kept in the refrigerator.
•
If FIASP FlexTouch Pens are stored at room temperature prior to first use, it should be used or thrown away within 28 days.
Pen in use:
•
Store the Pen you are currently using without the needle attached at room temperature below 86°F (30°C) or in the refrigerator at 36°F to 46°F (2°C to 8°C) for up to 28 days.
•
Keep FIASP away from excessive heat or light.
•
The FIASP FlexTouch Pen you are using is to be thrown away after 28 days, even if it still has insulin left in it and the expiration date has not passed.
General Information about the safe and effective use of FIASP:
•
**Keep FIASP FlexTouch Pens and needles out of the reach of children.**
•
**Always** use a new needle for each injection.
•
**Do not** share FIASP FlexTouch Pens or needles with other people. You may give other people a serious infection, or get a serious infection from them.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-800-727-6500
U.S. License No. 1261
Revised: 06/2023

For more information go to
© 2023 Novo Nordisk

INSTRUCTIONS FOR USE
FIASP®[fee’asp] PenFill****®
(insulin aspart)
injection, for subcutaneous use
3 mL cartridge: 100 units/mL (U-100)
•
**Do not share your PenFill cartridge or PenFill cartridge compatible insulin delivery device with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.**
•
Your healthcare provider should show you or your caregiver how to inject FIASP the right way before you inject it for the first time.
•
**FIASP PenFill****cartridge 100 units/mL is a prefilled single-patient-use cartridge** containing 300 units of U-100 FIASP (insulin aspart) injection.
•
After you insert the PenFill cartridge in your device, you can use it for multiple injections. Read the** instruction manual**that comes with your insulin delivery device for complete instructions on how to use the PenFill cartridge with the device.
•
This PenFill cartridge is not recommended for use by the blind or visually impaired without the assistance of a person trained in the proper use of the product and your insulin delivery device.
•
If using a** new**FIASP PenFill cartridge, start with** Step 1.**
•
If the FIASP PenFill cartridge has already been**used**, start with** Step 2.**
Supplies you will need to give your FIASP injection:
•
FIASP PenFill cartridge
•
Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery device
•
1 new NovoFine®, NovoFine® Plus, or NovoTwist® needle
•
Alcohol swab
•
Adhesive bandage
•
Cotton gauze
•
A sharps container for throwing away used PenFill cartridges and needles.**See “After your injection” at the end of these instructions.**
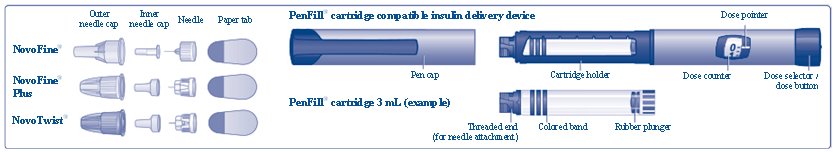
(Figure A)
How to use the FIASP PenFill cartridge
•
Wash your hands with soap and water.
•
Before you start to prepare your injection,**check the FIASP PenFill cartridge label**to make sure that it contains the insulin you need. This is especially important if you take more than 1 type of insulin.
•
The tamper-resistant foil should be in place before the first use. If the foil has been broken or removed before your first use of the cartridge,**do not** use it. Call Novo Nordisk at 1-800-727-6500.
•
Carefully look at the cartridge and the insulin inside it. Check that the FIASP cartridge:
o
is not damaged, for example cracked or leaking
o
is not loose on the threaded end
•
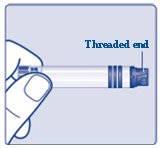
FIASP should look clear and colorless.**Do not** use FIASP if it is cloudy or colored or if the threaded end is loose (See Figure B).
(Figure B)
Step 1:
•
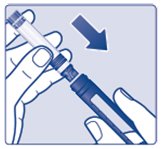
Insert a 3 mL cartridge with the threaded end first into your Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery device (See Figure C).
•
If you drop your device, check the insulin cartridge for damage such as cracks or leaking. If your cartridge is damaged, throw it away and use a new one.
(Figure C)
Prepare your device with a new needle
Step 2:
•
**Take a new needle,**and tear off the paper tab. Always use a new needle for each injection to make sure the needle is free of germs (sterile) and to prevent blocked needles.**Do not attach a new needle**to your device until you are ready to give your injection.**Do not reuse or share your needles with other people. You may give others a serious infection, or get a serious infection from them.**
•
Be careful not to bend or damage the needle before you use it.
•
Push the needle straight onto the device. Turn the needle clockwise until it is on tight (See Figure D).

(Figure D)
Step 3:
•
**Pull off the outer needle cap**(See Figure E).**Do not** throw it away. You will need it after the injection to safely remove the needle.
(Figure E)
Step 4:
•
**Pull off the inner needle cap**and throw it away (See Figure F).**Do not** try to put the inner needle cap back on the needle.
(Figure F)
A drop of insulin may appear at the needle tip. This is normal, but you must still check the insulin flow.
Check the insulin flow
Step 5:
•
Small amounts of air may collect in the cartridge during normal use. You must do an airshot before each injection to avoid injecting air and to make sure you receive the prescribed dose of your medicine.
•
Do the airshot as described in the instruction manual that comes with your device.
•
Keep testing your Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery device until you see insulin at the needle tip. If you still do not see a drop of insulin after 6 times, change the needle and repeat this step. This makes sure that any air bubbles are removed and that insulin is getting through the needle (See Figure G).

(Figure G)
Select your dose
Step 6:
•
**Check to make sure that the dose counter is set to 0.**
•
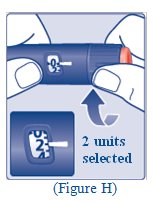
**Turn the dose selector clockwise to select the dose you need**to inject (See Figure H). The pointer should line up with your dose. When turning the dose selector, be careful not to press the dose button as insulin will come out. You will hear a click for every single unit dialed.**Do not** set the dose by counting the number of clicks you hear because you may get an incorrect dose.
•
Refer to your insulin delivery device manual if necessary.
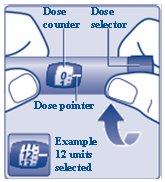
(Figure H)
Inject your dose
Step 7:
•
**Do the injection exactly as shown to you by your healthcare provider. Your healthcare provider should tell you if you need to pinch the skin before injecting.**
•
**You should take your dose of FIASP at the start of a meal or within 20 minutes after starting a meal.**
•
**FIASP can be injected under the skin (subcutaneously) of your stomach area (abdomen), upper legs (thighs), or upper arms (See Figure I).**
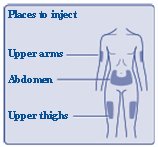
(Figure I)
•
**Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same injection site for each injection. Do not inject where the skin has pits, is thickened, or has lumps. Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.**
•
**Clean your injection site with an alcohol swab. Let your skin dry. Do not touch this area again before injecting.**
•
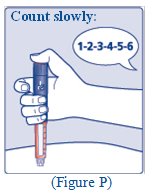
**Insert the needle into your skin. Press and hold down the dose button until the dose counter shows “0”. Continue to keep the dose button pressed and keep the needle in your skin and slowly count to 6 (see Figure J).**
•
**Remove the needle from your skin.**
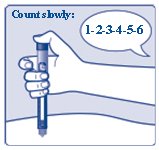
(Figure J)
You may see a drop of FIASP at the needle tip after injecting. This is normal and has no effect on the dose you just received. If blood appears after you take the needle out of your skin, press the injection site lightly with a cotton gauze and cover with an adhesive bandage, if necessary.Do not rub the area.
After your injection
Step 8:
•
Lay your outer needle cap on a flat surface. Carefully, lead the needle tip into the outer needle cap without touching the needle (See Figure K) and push the outer needle cap completely on.
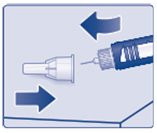
(Figure K)
•
Hold the black cartridge holder on the insulin delivery device and unscrew the needle counterclockwise (See Figure L).
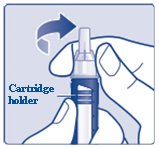
(Figure L)
•
Throw away (dispose of) the needle in an FDA-cleared sharps container as your healthcare professional has instructed you.
•
Put your empty FIASP PenFill cartridge and used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and PenFill cartridges in your household trash.
•
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
o
made of a heavy-duty plastic
o
can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
o
upright and stable during use
o
leak-resistant
o
properly labeled to warn of hazardous waste inside the container
•
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles or syringes with other people. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
•
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Step 9:
•
Keep the 3 mL PenFill cartridge in the device.**Do not** store your device with a needle attached. This will prevent infection or leakage of insulin and will make sure that you receive the right dose of FIASP.
•
**Put the pen cap on**your device after each use to protect the insulin from light (See Figure M).
(Figure M)
How should I store my FIASP PenFill cartridge?
Before use:
•
Store unused FIASP PenFill cartridges in the refrigerator at 36°F to 46°F (2°C to 8°C).
•
**Do not** freeze FIASP.**Do not** use FIASP if it has been frozen.
•
Unused PenFill cartridges may be used until the expiration date printed on the label, if kept in the refrigerator.
•
If FIASP is stored mistakenly outside of refrigeration between 47°F (9°C) to 86°F (30°C) prior to first use, it should be used within 28 days or thrown away.
PenFill cartridges in use:
•
Store the PenFill cartridge you are currently using in the insulin delivery device at room temperature below 86°F (30°C) for up to 28 days. Do not refrigerate.
•
Keep FIASP away from heat or light.
•
The FIASP PenFill cartridge you are using should be thrown away after 28 days, even if it still has insulin left in it.
General Information about the safe and effective use of FIASP.
•
**Keep FIASP PenFill cartridges and needles out of the reach of children.**
•
**Do not** share FIASP PenFill cartridges or needles with other people. You may give other people a serious infection, or get a serious infection from them.
•
**Always carry extra insulin of the same type(s) you use in case of loss or damage.**
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
© 2023 Novo Nordisk
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-800-727-6500
U.S. License No. 1261
Revised: 06/2023
INSTRUCTIONS FOR USE
FIASP®[fee’asp] PumpCart****®
(insulin aspart)
injection, for subcutaneous use
1.6 mL cartridge: 100 units/mL (U-100)
•
**Please read the pump manual (user guide)** that comes with your insulin pump.
•
Only use FIASP PumpCart with a compatible insulin pump. Check the insulin pump manual for instructions to see if FIASP PumpCart can be used with the pump. Do not use with other devices that are not designed for use with FIASP PumpCart.
•
FIASP PumpCart is ready for use directly in the pump. Using the wrong device may result in the wrong insulin dosing and lead to high blood sugar (hyperglycemia) or low blood sugar (hypoglycemia).
•
Do not share your PumpCart cartridge with other people. You may give other people a serious infection or get a serious infection from them.
•
Do not use the PumpCart cartridge if you are blind or visually impaired without the assistance of a person trained in the proper use of the PumpCart.
•
Do not mix with any other insulins.
•
Do not refill FIASP PumpCart. When it is empty, throw away (dispose) the cartridge.
•
Do not use FIASP PumpCart in an insulin pen.
Preparing and Using the FIASP PumpCart cartridge
•
Take FIASP PumpCart out of its package.
•
Check the label to make sure that it is FIASP PumpCart and confirm that you are using a compatible insulin pump.
•
Check the expiry date (EXP) – which is on the label and the carton.
•
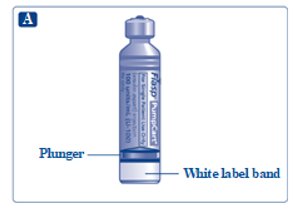
See picture A.**Do not use the cartridge**if any damage or leakage is seen, if the plunger has moved, or if the bottom of the plunger is visible above the white label band. This could be a result of leakage of insulin. Contact Novo Nordisk If you suspect that your FIASP PumpCart cartridge is damaged.
•
Check that the insulin in FIASP PumpCart is clear and colorless. If the insulin looks cloudy, do not use FIASP PumpCart. The cartridge may contain small bubbles.
•
Wash your hands with soap and water prior to installing the cartridge in your insulin pump.
•
Follow the instructions in the pump user manual to insert a new FIASP PumpCart cartridge in your pump and to remove the FIASP PumpCart cartridge from your pump.
•
Replace the FIASP PumpCart cartridge at least every 4 days, or according to the pump user manual, whichever is shorter, even if you have not used all of the insulin.
•
Change the infusion sets and the infusion set insertion site according to the insulin pump manufacturers’ user manual.
•
Throw away FIASP PumpCart in the pump reservoir if it has been exposed to temperatures higher than 98.6°F (37°C).
**How should I dispose my FIASP PumpCart cartridge?**
•
Put your empty FIASP PumpCart in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) Fiasp PumpCart in your household trash.
•
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
o
made of a heavy-duty plastic
o
can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
o
upright and stable during use
o
leak-resistant
o
properly labeled to warn of hazardous waste inside the container
•
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: <http://www.fda.gov/safesharpsdisposal>. Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store my FIASP PumpCart cartridge?
•
Not in-use (unopened) cartridges: Refrigerate at 36°F to 46°F (2°C to 8°C) in the original carton until expiration or for up to 18 days below 86°F (30°C).
•
In-use (opened) cartridge: Do not refrigerate. Keep below 98.6°F (37°C) for up to 4 days. The maximum time at room temperature is 18 days including 4 days in the pump.
•
Do not freeze.
•
Keep FIASP PumpCart cartridge away from heat or light.
General Information about the safe and effective use of FIASP PumpCart cartridge
•
Keep FIASP PumpCart out of the reach of children.
•
Always carry extra insulin of the same type you use in case of loss or damage**.**
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
© 2023 Novo Nordisk
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, New Jersey 08536
US License Number 1261
Revised: 06/2023
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
•
Always check insulin label before administration [see Warnings and Precautions (5.4)].
•
Inspect FIASP visually before use. It should appear clear and colorless. Do not use FIASP if particulate matter or coloration is seen.
•
Use FIASP FlexTouch pen with caution in patients with visual impairment who may rely on audible clicks to dial their dose.
•
Use PenFill cartridges with caution in patients with visual impairment.
•
Do**not** mix FIASP with any other insulin.
2.2 Route of Administration Instructions
Subcutaneous Injection:
•
Inject FIASP at the start of a meal or within 20 minutes after starting a meal subcutaneously into the abdomen, upper arm, or thigh.
•
Rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not inject into areas of lipodystrophy or localized cutaneous amyloidosis [see Adverse Reactions (6.1, 6.2)].
•
FIASP given by subcutaneous injection should generally be used in regimens with intermediate or long-acting insulin.
•
Instruct patients on basal-bolus treatment who forget a mealtime dose to monitor their blood glucose level to decide if an insulin dose is needed, and to resume their usual dosing schedule at the next meal.
•
The FIASP FlexTouch pen dials in 1 unit increments.
Continuous Subcutaneous Infusion (Insulin Pump):
•
Refer to the continuous subcutaneous insulin infusion pump user manual to see if FIASP or FIASP PumpCart can be used with the insulin pump. Use FIASP and FIASP PumpCart in accordance with the insulin pump system’s instructions for use.
•
Administer FIASP by continuous subcutaneous infusion in a region recommended in the instructions from the pump manufacturer. Rotate infusion sites within the same region to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not infuse into areas of lipodystrophy or localized cutaneous amyloidosis [see Warnings and Precautions (5.2) Adverse Reactions (6.1)].
•
Train patients using continuous subcutaneous insulin infusion therapy to administer insulin by injection and have alternate insulin therapy available in case of insulin pump failure [see Warnings and Precautions (5.8)].
•
Change FIASP in the pump reservoir at least every 6 days or replace the PumpCart cartridge at least every 4 days, or according to the pump user manual, whichever is shorter. Follow the FIASP-specific information for in-use time because FIASP-specific information may differ from general insulin pump user manual instructions.
•
Change the infusion sets and the infusion set insertion site according to the manufacturers user manual.
•
Do not mix with other insulins or diluents in the insulin pump.
•
Do not expose FIASP in the pump reservoir to temperatures greater than 37°C (98.6°F).
Intravenous Administration:
•
Administer FIASP intravenously**only** under medical supervision with close monitoring of blood glucose and potassium levels to avoid hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.5)].
•
Dilute FIASP to concentrations from 0.5 unit/mL to 1 unit/mL insulin aspart in infusion systems using polypropylene infusion bags.
•
FIASP is stable at room temperature at 20°C to 25°C (68°F to 77°F) for 24 hours in 0.9% sodium chloride or 5% dextrose infusion fluids.
2.3 Dosage Recommendations
•
Individualize the dosage of FIASP based on the route of administration, patient’s metabolic needs, blood glucose monitoring results, and glycemic control goal.
•
If converting from another mealtime insulin to FIASP, the initial change can be done on a unit-to-unit basis.
•
Dose adjustments may be needed when switching from another insulin, with changes in physical activity, changes in concomitant medications, changes in meal patterns (i.e., macronutrient content or timing of food intake), changes in renal or hepatic function or during acute illness to minimize the risk of hypoglycemia or hyperglycemia [see Warnings and Precautions (5.2, 5.3) and Drug Interactions (7), and Use in Specific Populations (8.6, 8.7)].
•
Closely monitor blood glucose when converting insulins used in insulin pumps as individualization of insulin pump parameters may be necessary to minimize the risk of hypoglycemia and hyperglycemia [see Warnings and Precautions (5.2, 5.3) and Adverse Reactions (6.1)]
•
During changes to a patient’s insulin regimen, increase the frequency of blood glucose monitoring [see Warnings and Precautions (5.2)].
•
Dosage adjustment may be needed when FIASP is co-administered with certain drugs [see Drug Interactions (7)].
•
Individualize and adjust the dosage of FIASP based on route of administration, individual’s metabolic needs, blood glucose monitoring results and glycemic control goal (2.3).
•
Dosage adjustments may be needed when switching from another insulin, with changes in physical activity, changes in concomitant medications, changes in meal patterns, changes in renal or hepatic function or during acute illness (2.3).
•
Subcutaneous injection (2.2):
o
Inject at the start of a meal or within 20 minutes after starting a meal into the abdomen, upper arm, or thigh.
o
Rotate injection sites within the same region to reduce the risk of lipodystrophy and localized cutaneous amyloidosis.
o
Should generally be used in regimens with an intermediate- or long-acting insulin.
•
Continuous Subcutaneous Infusion (Insulin Pump) (2.2):
o
Refer to the insulin infusion pump user manual to see if FIASP can be used. Use in accordance with the insulin pumps’ instructions for use.
o
Administer by continuous subcutaneous infusion using an insulin pump in a region recommended in the instructions from the pump manufacturer. Rotate infusion sites within the same region to reduce the risk of lipodystrophy and localized cutaneous amyloidosis.
•
Intravenous Infusion: Administer only under medical supervision after diluting to concentrations from 0.5 to 1 unit/mL insulin aspart in infusion systems using polypropylene infusion bags (2.2).