Helium Oxygen mixture
Helium Oxygen Mixture
ef29f856-4675-4bb7-adef-67d7c6f27439
HUMAN PRESCRIPTION DRUG LABEL
Jan 19, 2023
Praxair Distribution, Inc.
DUNS: 042845636
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Helium Oxygen mixture
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
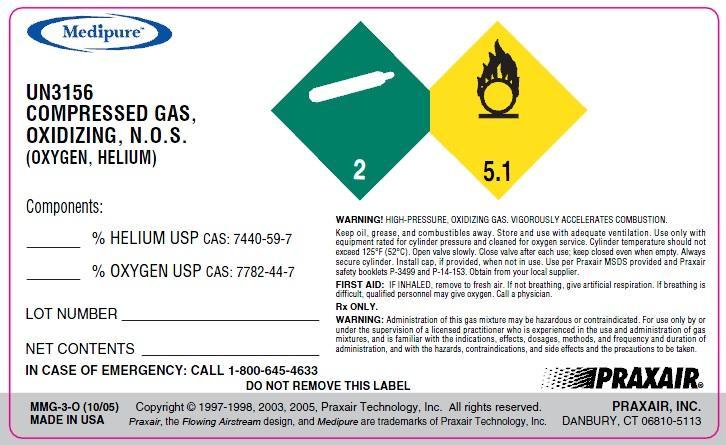
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
UN3156
COMPRESSED GAS,
OXIDIZING, N.O.S.
(OXYGEN, HELIUM)
% HELIUM USP CAS: 7440-59-7
% OXYGEN USP CAS: 7782-44-7
NET CONTENTS
LOT NUMBER
WARNING! HIGH-PRESSURE, OXIDIZING GAS. VIGOROUSLY ACCELERATES COMBUSTION.
Keep oil, grease, and combustibles away. Store and use with adequate ventilation. Use only with equipment rated for cylinder pressure and cleaned for oxygen service. Cylinder temperature should not exceed 125°F (52°C). Open valve slowly. Close valve after each use; keep closed even when empty. Always secure cylinder. Install cap, if provided, when not in use. Use a backflow prevention device in any piping. Use per MSDS provided and Praxair safety booklets P-3499 and P-14-153. Obtain from your local supplier.
FIRST AID: IF INHALED, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, qualified personnel may give oxygen. Call a physician.
RX ONLY
WARNING! Administration of this gas mixture may be hazardous or contraindicated. For use only by or under the supervision of a licensed
practitioner who is experienced in the use and administration of gas mixtures and is familiar with the indications, effects, dosages, methods and
frequency and duration of administration, and with the hazards, contraindications, and side effects and the precautions to be taken.
Rx only IN CASE OF EMERGENCY CALL 1-800-645-4633
Copyright 1997-1998, 2003, 2005 Praxair Technology, Inc.
DO NOT REMOVE THIS LABEL MMG-3-0 (10/05)
Praxair, the flowing airstream design and Medipure are trademarks of Praxair
Technology, Inc. DISTRIBUTED BY PRAXAIR, INC, DANBURY, CT 06810-5113