ERWCNMIE Toenail Fungus Treatment Pen
83766-108 ERWCNMIE Toenail Fungus Treatment Pen
3dce0c33-d4f3-5135-e063-6394a90a85a6
HUMAN OTC DRUG LABEL
Sep 2, 2025
Shenzhen Joyuflsh Technology Co.,Ltd.
DUNS: 603012375
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Tolnaftate, UNDECYLENIC ACID
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
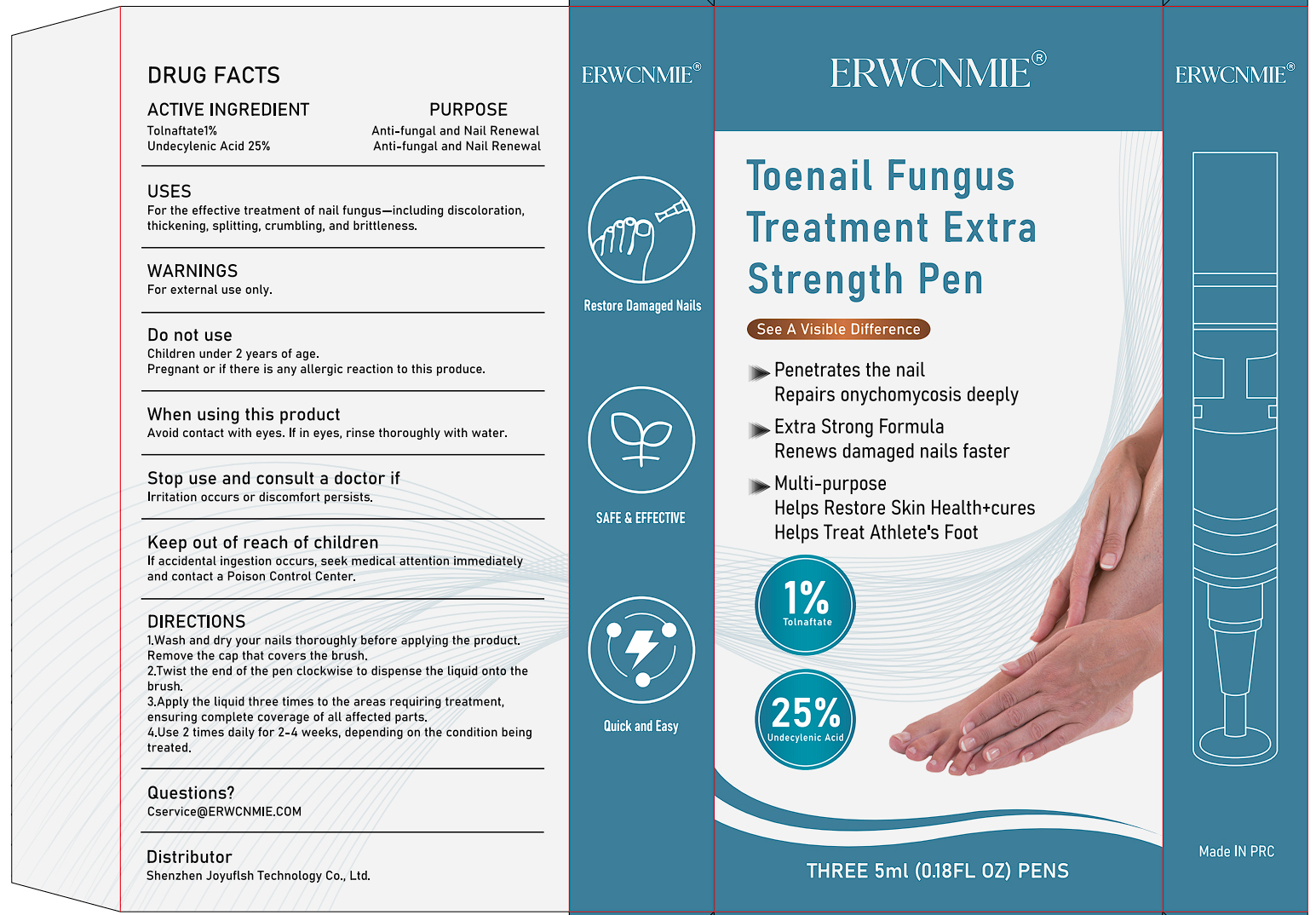
INDICATIONS & USAGE SECTION
For the effective treatment of nail fungus-including discoloration,.
thickening, splitting, crumbling, and brittleness.
OTC - ACTIVE INGREDIENT SECTION
Tolnaftate 1%
UNDECYLENIC ACID 25%
OTC - PURPOSE SECTION
Anti-fungal and Nail Renewal
WARNINGS SECTION
For external use only.
OTC - DO NOT USE SECTION
Children under 2 years of age.
Pregnant or if there is any allergic reaction to this produce.
OTC - WHEN USING SECTION
Avoid contact with eyes. lf in eyes, rinse thoroughly with water.
OTC - STOP USE SECTION
lrritation occurs or discomfort persists.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
If accidental ingestion occurs, seek medical attention immediatelyand contact a Poison Control Center.
DOSAGE & ADMINISTRATION SECTION
1.Wash and dry your nails thoroughly before applying the product.Remove the cap that covers the brush.
2.Twist the end of the pen clockwise to dispense the liquid onto thebrush.
3.Apply the liquid three times to the areas requiring treatment,ensuring
complete coverage of all affected parts.
4.Use 2 times daily for 2-4 weeks, depending on the condition beingtreated.
INACTIVE INGREDIENT SECTION
ALCOHOL
POLYETHYLENE GLYCOL
PROPYLENE GLYCOL
DIMETHICONE
C12-15 ALKYL LACTATE
ETHYLHEXYLGLYCERIN
GLYCERIN
LAUROCAPRAM
SOPHORA FLAVESCENS ROOT
TOCOPHEROL
PHENOXYETHANOL
WATER
