PM Toothache Relief
5820623 Walgreens Nightime 4X Medicated Cream (8015623)
e816f742-fba9-3c12-e053-2a95a90a192f
HUMAN OTC DRUG LABEL
Aug 12, 2025
Walgreens
DUNS: 008965063
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Benzocaine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
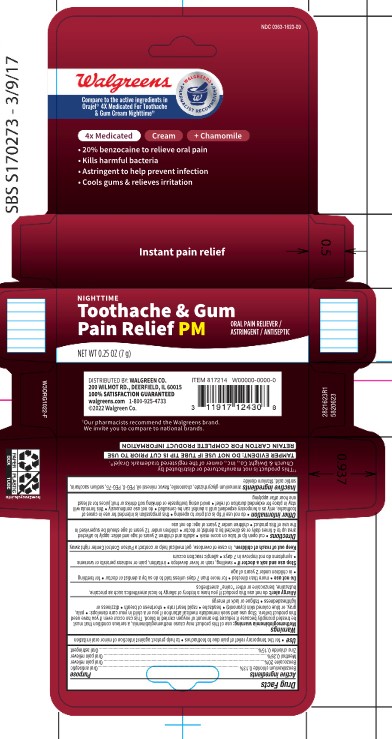
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
20% benzocaine to releive oral pain
Kills harmful bacteria
Astringent to help prevent infection
Cools gums and relieves irritation
Net Wt 0.25 oz (7g)
INDICATIONS & USAGE SECTION
Cut open tip of tube on score mark. Adults and children 2 years of age and older: apply to affected area up to 4 times daily or as directed by a dentist or doctor. Children under 12 years of age should be supervised in the use of this product. Children under 2 years of age: do not use.
DOSAGE & ADMINISTRATION SECTION
For the temporary relief of pain due to toothaches. To help protect aganist infection of minor oral irritation.
INACTIVE INGREDIENT SECTION
ammonium glycyrrhizate, chamomile, flavor, mineral oil, PEG-8, PEG-77, sodium saccharin, sorbic acid, titanium dioxide
OTC - ACTIVE INGREDIENT SECTION
Belzalkomium Chloride 0.13% Oral antiseptic
Benzocaine 20% Oral pain releiver
Methol 0.26% Oral pain releiver
Zinc chloride 0.15% Oral astringent
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. In case of overdose, get medical help or contact a POison Control Center right away.
OTC - PURPOSE SECTION
Do not nuse if tube tip is cust prior to opening. This preparation is intended for use in cases of toothache, only as a temporary expedient until a dentist can be consulted. Do not use continuously . This formula will stay in place for extended duration of relief. Avoid using toothpaste or drinking soft drinks or fruit juices for at least one hour after applying.
WARNINGS SECTION
Methemoglobinemia warning: use of this product may cause methemoglobinemia, a serious condition tha tmust be treated promptly becuase it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops: pale, gray, or blue colored skin (cyanosis), headache, rapid heart rate, shortness of breath, dizziness or lightheadedness, fatigue or lack of energy.
