Cough DM
Meijer Distribution, Inc. Cough DM Drug Facts
2f733ad1-b6f2-ebab-e063-6294a90abb9e
HUMAN OTC DRUG LABEL
Sep 15, 2025
Sixarp, LLC
DUNS: 016329513
Praxis, LLC
DUNS: 016329513
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
dextromethorphan polistirex
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (19)
Drug Labeling Information
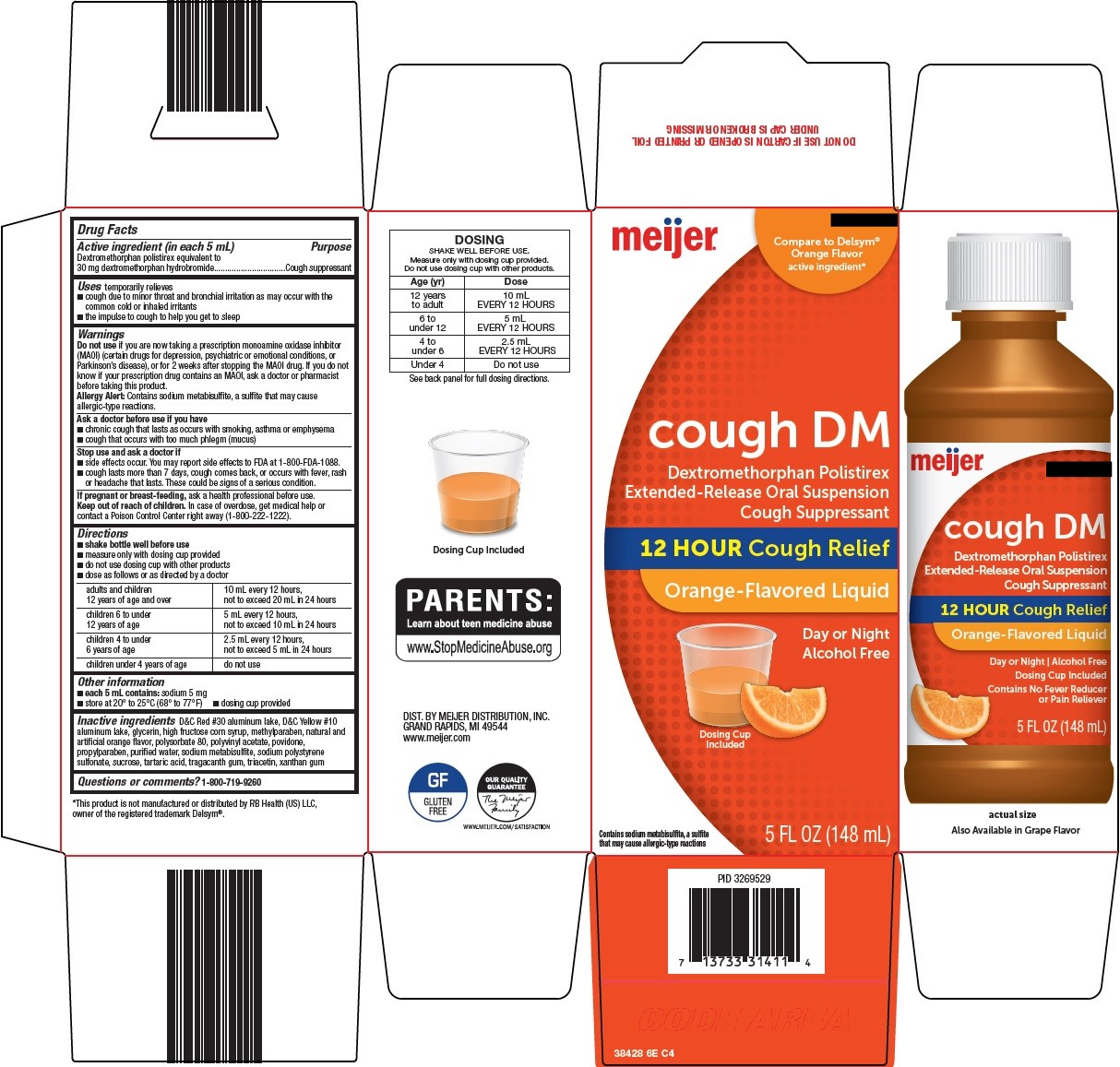
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Principal Display Panel
Compare to Delsym® Orange Flavor active ingredient
cough DM
Dextromethorphan Polistirex
Extended-Release Oral Suspension
Cough Suppressant
12 HOUR Cough Relief
Orange-Flavored Liquid
Day or Night
Alcohol Free
Dosing Cup Included
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions
5 FL OZ (148 mL)