Dry eye relief
Sterile Dry eye Relief
f8da3af5-9172-4023-be82-0fd5bd891616
HUMAN OTC DRUG LABEL
Sep 12, 2025
CARDINAL HEALTH 110, LLC. DBA LEADER
DUNS: 063997360
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
polyethylene glycol and propylene glycol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
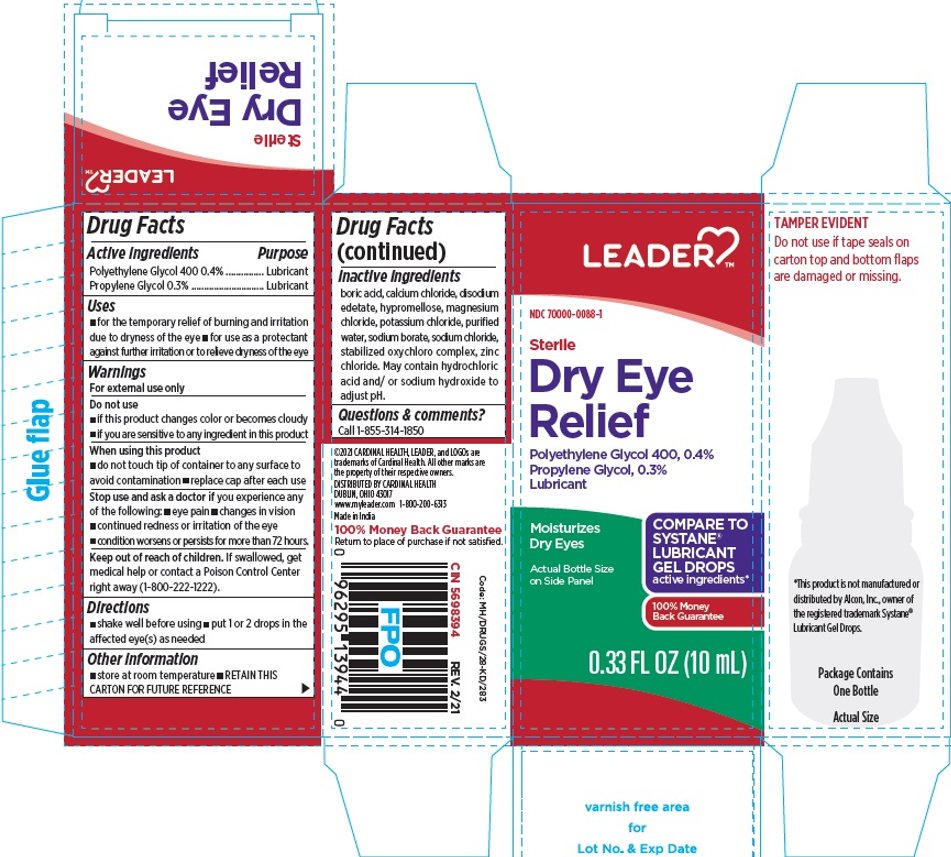
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
LEADER®
NDC 70000-0088-1
Sterile
Dry Eye
Relief
Polyethylene Glycol 400, 0.4%
Propylene Glycol, 0.3%
Lubricant
Moisturizes Dry Eyes
Actual Bottle Size on Side Panel
COMPARE TO SYSTANE® LUBRICANT GEL DROPS active ingredients*
100% Money Back Guarantee
0.33 FL OZ (10 mL)
INDICATIONS & USAGE SECTION
Uses
▪
for the temporary relief of burning and irritation due to dryness of the eye
▪
for use as a protectant against further irritation or to relieve dryness of the eye
OTC - ACTIVE INGREDIENT SECTION
|
Active Ingredients |
Purpose |
|
Polyethylene Glycol 400 0.4% |
Lubricant |
|
Propylene Glycol 0.3% |
Lubricant |
WARNINGS SECTION
Warnings
For external use only
OTC - DO NOT USE SECTION
Do not use
▪
if this product changes color or becomes cloudy
▪
if you are sensitive to any ingredient in this product
DOSAGE & ADMINISTRATION SECTION
Directions
▪
shake well before using
▪
put 1 or 2 drops in the affected eye(s) as needed
OTC - WHEN USING SECTION
When using this product
▪
do not touch tip of container to any surface to avoid contamination
▪
replace cap after each use
OTC - STOP USE SECTION
Stop use and ask a doctor ifyou experience any of the following:
▪
you feel eye pain
▪
changes in vision
▪
continued redness or irritation of the eye
▪
condition worsens or persists for more than 72 hours
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
**Keep out of reach of children.**If swallowed, get medical help or contact a Poison Control Center right away.
INACTIVE INGREDIENT SECTION
Inactive ingredients
boric acid, edetate disodium , potassium chloride, mangnesium chloride , sodium chloride, sodium borate , purified water. May contain hydrochloric acid and/or sodium hydroxide to adjust pH.
OTHER SAFETY INFORMATION
Other information
▪
store at room temperature
SPL UNCLASSIFIED SECTION
.
TAMPER EVIDENT
Do not use if tape seals on carton top and bottom flaps are damaged or missing.
*This product is not manufactured or distributed by Alcon, Inc., owner of the registered trademark Systane® Lubricant Gel Drops.
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com 1-800-200-9313
Made in India
100% Money back Guarantee
Return to place of purchase if not satisfied.
Code: MH/DRUGS/28-KD/283
|
CIN 5698394 |
REV. 2/21 |
