Medroxyprogesterone Acetate
These highlights do not include all the information needed to use MEDROXYPROGESTERONE ACETATE INJECTABLE SUSPENSION safely and effectively. See full prescribing information for MEDROXYPROGESTERONE ACETATE INJECTABLE SUSPENSION. MEDROXYPROGESTERONE ACETATE injectable suspension, for intramuscular useInitial U.S. Approval: 1959
d4d27703-5ad0-4ead-9481-0aba2fa1b45c
HUMAN PRESCRIPTION DRUG LABEL
Mar 15, 2023
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Medroxyprogesterone Acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Medroxyprogesterone acetate injectable suspension should not be administered during pregnancy [see Contraindications (4) and Warnings and Precautions (5.17)].
8.3 Nursing Mothers
Detectable amounts of drug have been identified in the milk of mothers receiving medroxyprogesterone acetate injectable suspension [see Warnings and Precautions (5.13)].
8.4 Pediatric Use
Medroxyprogesterone acetate injectable suspension is not indicated before menarche. Use of medroxyprogesterone acetate injectable suspension is associated with significant loss of BMD. This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity. It is unknown if use of medroxyprogesterone acetate injectable suspension by younger women will reduce peak bone mass and increase the risk of osteoporotic fractures in later life. Other than concerns about loss of BMD, the safety and effectiveness are expected to be the same for postmenarchal adolescents and adult women.
8.5 Geriatric Use
This product has not been studied in post-menopausal women and is not indicated in this population.
8.6 Renal Impairment
The effect of renal impairment on medroxyprogesterone acetate injectable suspension pharmacokinetics has not been studied.
8.7 Hepatic Impairment
The effect of hepatic impairment on medroxyprogesterone acetate injectable suspension pharmacokinetics has not been studied. Medroxyprogesterone acetate injectable suspension should not be used by women with significant liver disease and should be discontinued if jaundice or disturbances of liver function occur [see Contraindications (4) and Warnings and Precautions (5.7)].
- Nursing Mothers: Detectable amounts of drug have been identified in the milk of mothers receiving medroxyprogesterone acetate injectable suspension. (8.3)
- Pediatric Patients: medroxyprogesterone acetate injectable suspension is not indicated before menarche. (8.4)
DESCRIPTION SECTION
11 DESCRIPTION
Medroxyprogesterone acetate injectable suspension, USP a contraceptive injection, contains medroxyprogesterone acetate, USP a derivative of progesterone, as its active ingredient. Medroxyprogesterone acetate is active by the parenteral and oral routes of administration. It is a white to off- white, odorless, fine powder that is stable in air and that melts between 200°C and 210°C. It is freely soluble in chloroform, soluble in acetone and dioxane, sparingly soluble in alcohol and methanol, slightly soluble in ether, and insoluble in water.
The chemical name for medroxyprogesterone acetate, USP is pregn-4-ene-3, 20-dione, 17-(acetyloxy)-6-methyl-, (6α)-. The structural formula is as follows:
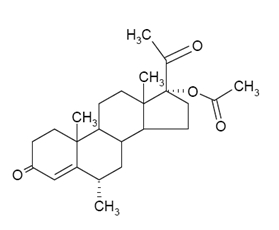
Molecular Formula C24H34O4 Molecular Weight 386.52
Medroxyprogesterone acetate injectable suspension, USP for IM injection is available in vials containing 1 mL of medroxyprogesterone acetate sterile aqueous suspension 150 mg/mL.
Each mL of sterile aqueous suspension contains:
|
Medroxyprogesterone acetate, USP |
150 mg |
|
Polyethylene glycol 3350 |
28.9 mg |
|
Polysorbate 80 |
2.41 mg |
|
Sodium chloride |
8.68 mg |
|
Methylparaben |
1.37 mg |
|
Propylparaben |
0.150 mg |
|
Water for injection |
quantity sufficient |
When necessary, pH is adjusted with sodium hydroxide or hydrochloric acid, or both.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Medroxyprogesterone acetate injectable suspension, USP is available as:
|
NDC Number |
Concentration |
Packacge Size |
|
63629-8744-01 |
150 mg/mL |
1 mL single-dose vials |
|
packaged individually |
Vials MUST be stored upright. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Medroxyprogesterone acetate injectable suspension [MPA] inhibits the secretion of gonadotropins which primarily prevents follicular maturation and ovulation and causes thickening of cervical mucus. These actions contribute to its contraceptive effect.
12.2 Pharmacodynamics
No specific pharmacodynamic studies were conducted with medroxyprogesterone acetate injectable suspension.
12.3 Pharmacokinetics
Absorption
Following a single 150 mg IM dose of medroxyprogesterone acetate injectable suspension in eight women between the ages of 28 and 36 years old, medroxyprogesterone acetate concentrations, measured by an extracted radioimmunoassay procedure, increase for approximately 3 weeks to reach peak plasma concentrations of 1 to 7 ng/mL.
Distribution
Plasma protein binding of MPA averages 86%. MPA binding occurs primarily to serum albumin. No binding of MPA occurs with sex-hormone-binding globulin (SHBG).
Metabolism
MPA is extensively metabolized in the liver by P450 enzymes. Its metabolism primarily involves ring A and/or side-chain reduction, loss of the acetyl group, hydroxylation in the 2-, 6-, and 21-positions or a combination of these positions, resulting in more than 10 metabolites.
Excretion
The concentrations of medroxyprogesterone acetate decrease exponentially until they become undetectable (<100 pg/mL) between 120 to 200 days following injection. Using an unextracted radioimmunoassay procedure for the assay of medroxyprogesterone acetate in serum, the apparent half-life for medroxyprogesterone acetate following IM administration of medroxyprogesterone acetate injectable suspension is approximately 50 days. Most medroxyprogesterone acetate metabolites are excreted in the urine as glucuronide conjugates with only minor amounts excreted as sulfates.
Specific Populations
The effect of hepatic and/or renal impairment on the pharmacokinetics of medroxyprogesterone acetate injectable suspension is unknown.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Contraception
In five clinical studies using medroxyprogesterone acetate injectable suspension, the 12-month failure rate for the group of women treated with medroxyprogesterone acetate injectable suspension was zero (no pregnancies reported) to 0.7 by Life-Table method. The effectiveness of medroxyprogesterone acetate injectable suspension is dependent on the patient returning every 3 months (13 weeks) for reinjection.
14.2 Bone Mineral Density Changes in Adult Women Treated with
Medroxyprogesterone Acetate Injectable Suspension
In a controlled, clinical study, adult women using medroxyprogesterone acetate injectable suspension (150 mg) for up to 5 years showed spine and hip bone mineral density (BMD) mean decreases of 5 to 6%, compared to no significant change in BMD in the control group. The decline in BMD was more pronounced during the first two years of use, with smaller declines in subsequent years. Mean changes in lumbar spine BMD of -2.86%, -4.11%, -4.89%, -4.93% and -5.38% after 1, 2, 3, 4, and 5 years, respectively, were observed. Mean decreases in BMD of the total hip and femoral neck were similar.
After stopping use of medroxyprogesterone acetate injectable suspension, there was partial recovery of BMD toward baseline values during the 2-year post- therapy period. Longer duration of treatment was associated with less complete recovery during this 2-year period following the last injection. Table 4 shows the change in BMD in women after 5 years of treatment with medroxyprogesterone acetate injectable suspension and in women in a control group, as well as the extent of recovery of BMD for the subset of the women for whom 2-year post treatment data were available.
Table 4. Mean Percent Change from Baseline in BMD in Adults by Skeletal Site and Cohort (5 Years of Treatment and 2 Years of Follow-Up)
|
Spine |
Total Hip |
Femoral Neck | ||||
|
Time in Study |
Medroxyprogesterone acetate injectable suspension***** |
Control****** |
Medroxyprogesterone acetate injectable |
Control****** |
Medroxyprogesterone acetate injectable suspension***** |
Control****** |
|
5 years |
-5.38% n = 33 |
0.43% n = 105 |
-5.16% n = 21 |
0.19% n = 65 |
-6.12% n = 34 |
-0.27% n = 106 |
|
7 years |
-3.13% n = 12 |
0.53% n = 60 |
-1.34% n = 7 |
0.94% n = 39 |
-5.38% n = 13 |
-0.11% n = 63 |
** The control group consisted of women who did not use hormonal contraception and were followed for 7 years. |
14.3 Bone Mineral Density Changes in Adolescent Females (12 to 18 Years of
Age) Treated with Medroxyprogesterone Acetate Injectable Suspension
The impact of medroxyprogesterone acetate injectable suspension (150 mg) use for up to 240 weeks (4.6 years) was evaluated in an open-label non-randomized clinical study in 389 adolescent females (12 to 18 years of age). Use of medroxyprogesterone acetate injectable suspension was associated with a significant decline from baseline in BMD.
Partway through the trial, drug administration was stopped (at 120 weeks). The mean number of injections per medroxyprogesterone acetate injectable suspension user was 9.3. Table 5 summarizes the study findings. The decline in BMD at total hip and femoral neck was greater with longer duration of use. The mean decrease in BMD at 240 weeks was more pronounced at total hip (-6.4%) and femoral neck (-5.4%) compared to lumbar spine (-2.1%).
Adolescents in the untreated cohort had an increase in BMD during the period of growth following menarche. However, the two cohorts were not matched at baseline for age, gynecologic age, race, BMD and other factors that influence the rate of acquisition of BMD.
Table 5.BMDMean Percent Change from Baseline in Adolescents Receiving ≥4 Injections per 60-week Period, by Skeletal Site and Cohort
|
Medroxyprogesterone acetate injectable suspension (150 mg IM) |
Unmatched, Untreated Cohort | |||
|
Duration of Treatment |
N |
Mean % Change |
N |
Mean % Change |
|
Total Hip BMD Week 60 (1.2 years) Week 240 (4.6 years) |
113 73 28 |
-2.75 -5.40 -6.40 |
166 109 84 |
1.22 2.19 1.71 |
|
Femoral Neck BMD Week 120 Week 240 |
113 73 28 |
-2.96 -5.30 -5.40 |
166 108 84 |
1.75 2.83 1.94 |
|
Lumbar Spine BMD Week 120 Week 240 |
114 73 27 |
-2.47 -2.74 -2.11 |
167 109 84 |
3.39 5.28 6.40 |
BMD Recovery Post-Treatment in Adolescents
Longer duration of treatment and smoking were associated with less recovery of BMD following the last injection of medroxyprogesterone acetate injectable suspension. Table 6 shows the extent of recovery of BMD up to 60 months post- treatment for adolescents who received medroxyprogesterone acetate injectable suspension for two years or less compared to more than two years. Post- treatment follow-up showed that, in women treated for more than two years, only lumbar spine BMD recovered to baseline levels after treatment was discontinued. Adolescents treated with medroxyprogesterone acetate injectable suspension for more than two years did not recover to their baseline BMD level at femoral neck and total hip even up to 60 months post-treatment. Adolescents in the untreated cohort gained BMD throughout the trial period (data not shown) [see Warnings and Precautions (5.1)].
Table 6. BMD Recovery (Months Post-Treatment) in Adolescents by Years of Medroxyprogesterone Acetate Injectable Suspension Use (2 Years or Less vs. More than 2 Years)
|
Duration of Treatment |
2 years or less |
More than 2 years | ||
|
N |
Mean % Change from baseline |
N |
Mean % Change from baseline | |
|
Total Hip BMD | ||||
|
End of Treatment |
49 |
-1.5% |
49 |
-6.2% |
|
12 M post-treatment |
33 |
-1.4% |
24 |
-4.6% |
|
24 M post-treatment |
18 |
0.3% |
17 |
-3.6% |
|
36 M post-treatment |
12 |
2.1% |
11 |
-4.6% |
|
48 M post-treatment |
10 |
1.3% |
9 |
-2.5% |
|
60 M post-treatment |
3 |
0.2% |
2 |
-1.0% |
|
Femoral Neck BMD | ||||
|
End of Treatment |
49 |
-1.6% |
49 |
-5.8% |
|
12 M post-treatment |
33 |
-1.4% |
24 |
-4.3% |
|
24 M post-treatment |
18 |
0.5% |
17 |
-3.8% |
|
36 M post-treatment |
12 |
1.2% |
11 |
-3.8% |
|
48 M post-treatment |
10 |
2.0% |
9 |
-1.7% |
|
60 M post-treatment |
3 |
1.0% |
2 |
-1.9% |
|
Lumbar Spine BMD | ||||
|
End of Treatment |
49 |
-0.9% |
49 |
-3.5% |
|
12 M post-treatment |
33 |
0.4% |
23 |
-1.1% |
|
24 M post-treatment |
18 |
2.6% |
17 |
1.9% |
|
36 M post-treatment |
12 |
2.4% |
11 |
0.6% |
|
48 M post-treatment |
10 |
6.5% |
9 |
3.5% |
|
60 M post-treatment |
3 |
6.2% |
2 |
5.7% |
14.4 Bone Fracture Incidence in Women Treated with Medroxyprogesterone
Acetate Injectable Suspension
A retrospective cohort study to assess the association between medroxyprogesterone acetate injectable suspension injection and the incidence of bone fractures was conducted in 312,395 female contraceptive users in the UK. The incidence rates of fracture were compared between medroxyprogesterone acetate injectable suspension users and contraceptive users who had no recorded use of medroxyprogesterone acetate injectable suspension. The Incident Rate Ratio (IRR) for any fracture during the follow-up period (mean = 5.5 years) was 1.41 (95% CI 1.35, 1.47). It is not known if this is due to medroxyprogesterone acetate injectable suspension use or to other related lifestyle factors that have a bearing on fracture rate.
In the study, when cumulative exposure to medroxyprogesterone acetate injectable suspension was calculated, the fracture rate in users who received fewer than 8 injections was higher than that in women who received 8 or more injections. However, it is not clear that cumulative exposure, which may include periods of intermittent use separated by periods of non-use, is a useful measure of risk, as compared to exposure measures based on continuous use.
There were very few osteoporotic fractures (fracture sites known to be related to low BMD) in the study overall, and the incidence of osteoporotic fractures was not found to be higher in medroxyprogesterone acetate injectable suspension users compared to non-users.
Importantly, this study could not determine whether use of medroxyprogesterone acetate injectable suspension has an effect on fracture rate later in life.
