Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**Dosage and Administration** **Posology** The primary vaccination schedule consists of three doses (of 0.5 ml) which should be administered according to official recommendations (see _Pharmacodynamics_ for schedules evaluated in clinical trials – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Infanrix hexa can be considered for the booster if the antigen composition is in accordance with the official recommendations. 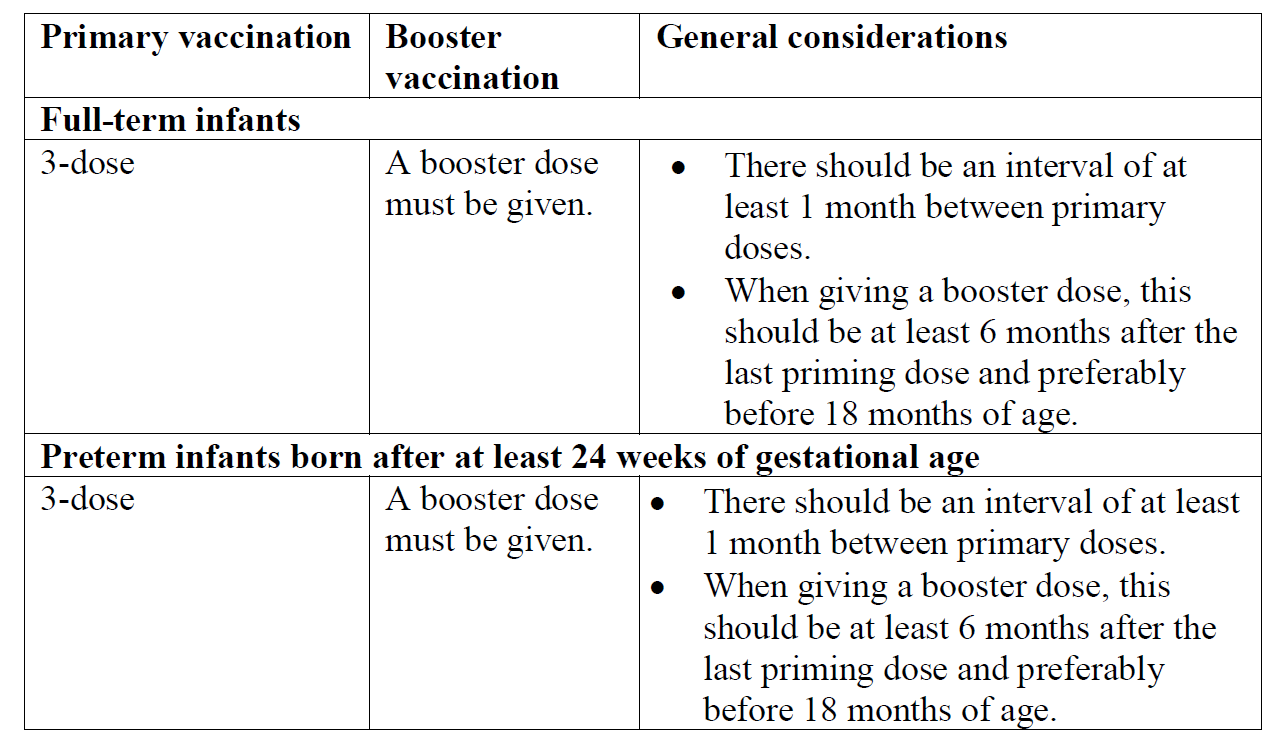 The vaccine may be used to complete a vaccination course initiated with Infanrix-IPV+Hib vaccine. During the development of the vaccine, the following primary vaccination schedules which are compatible with a policy of administering hepatitis B vaccine at birth were studied in full-term infants: at 2, 3, 4 months of age; at 2, 4, 6 months of age; at 1.5, 3, 5 months of age and at 6, 10, 14 weeks of age. Additionally, in a study in full-term infants undertaken in Singapore, Infanrix hexa was given as part of a 3, 4, 5 month schedule with Infanrix-IPV+Hib. The Infanrix-IPV+Hib was administered at months 3 and 4 and Infanrix hexa given at month 5. As part of this study, monovalent Hepatitis B vaccine was given at 0 and 1 month in accord with Singapore’s Hepatitis B immunisation policy. Immunogenicity data for preterm infants was solely obtained in trials that studied 2-4-6 months schedule for primary vaccination. Safety data for preterm infants was also obtained in study Rota-54 that included a greater variety of schedules, i.e. in France (2-3-4 months), Poland (2-3/4-5 months), Spain and Portugal (2-4-6 months). The Expanded Program on Immunisation schedule (at 6, 10, 14 weeks of age) may only be used if a dose of hepatitis B vaccine has been given at birth. Where a dose of hepatitis B vaccine is given at birth, Infanrix hexa can be used as a replacement for supplementary doses of hepatitis B vaccine from the age of 6 weeks. If a second dose of hepatitis B vaccine is required before this age, monovalent hepatitis B vaccine should be used. Locally established immunoprophylactic measures against hepatitis B should be maintained. **Method of administration** Infanrix hexa is for deep intramuscular injection.
INTRAMUSCULAR
Medical Information
**Indications** Infanrix hexa is indicated for primary and booster vaccination of infants and toddlers against diphtheria, tetanus, pertussis, hepatitis B, poliomyelitis and _Haemophilus influenzae_ type b. The use of Infanrix hexa should be in accordance with official recommendations.
**Contraindications** Hypersensitivity to the active substances or to any of the excipients or residues such as formaldehyde, neomycin and polymyxin (see _Qualitative and quantitative composition_ and _List of Excipients_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Hypersensitivity after previous administration of diphtheria, tetanus, pertussis, hepatitis B, polio or Hib vaccines. Infanrix hexa is contraindicated if the child has experienced an encephalopathy of unknown aetiology, occurring within 7 days following previous vaccination with pertussis-containing vaccine. In these circumstances, pertussis vaccination should be discontinued and the vaccination course should be continued with diphtheria-tetanus, hepatitis B, inactivated polio and Hib vaccines.
J07CA09
diphtheria-haemophilus influenzae B-pertussis-poliomyelitis-tetanus-hepatitis B
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
GLAXOSMITHKLINE BIOLOGICALS SA
GlaxoSmithKline Biologicals
GSK Biologicals SA
Active Ingredients
HIB PURIFIED CAPSULAR POLYSACCHARIDE BOUND TO TETANUS TOXOID
10 mcg/0.5 ml
DIPHTHERIA TOXOID
min 30 iu/0.5 ml
FILAMENTOUS HAEMAGGLUTININ
25 mcg/0.5 ml
PERTUSSIS TOXOID
25 mcg/0.5 ml
Documents
Package Inserts
Infanrix Hexa PI.pdf
Approved: May 31, 2023
