Sinus Tonic
DRUG FACTS:
63234fdc-7456-42c6-8996-fef372dd4a53
HUMAN OTC DRUG LABEL
May 20, 2025
BioActive Nutritional, Inc.
DUNS: 624980496
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Echinacea (Angustifolia), Baptisia Tinctoria, Thiaminum Hydrochloricum, Hydrastis Canadensis, Pyridoxinum Hydrochloricum, Spongia Tosta, Apis Mellifica, Ascorbic Acid, Hepar Sulphuris Calcareum, Kali Bichromicum, Lachesis Mutus, Mercurius Iodatus Ruber, Mercurius Sulphuratus Ruber, Pulsatilla (Pratensis), Silicea, Sinusitisinum, Pantothenic Acid
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (19)
Drug Labeling Information
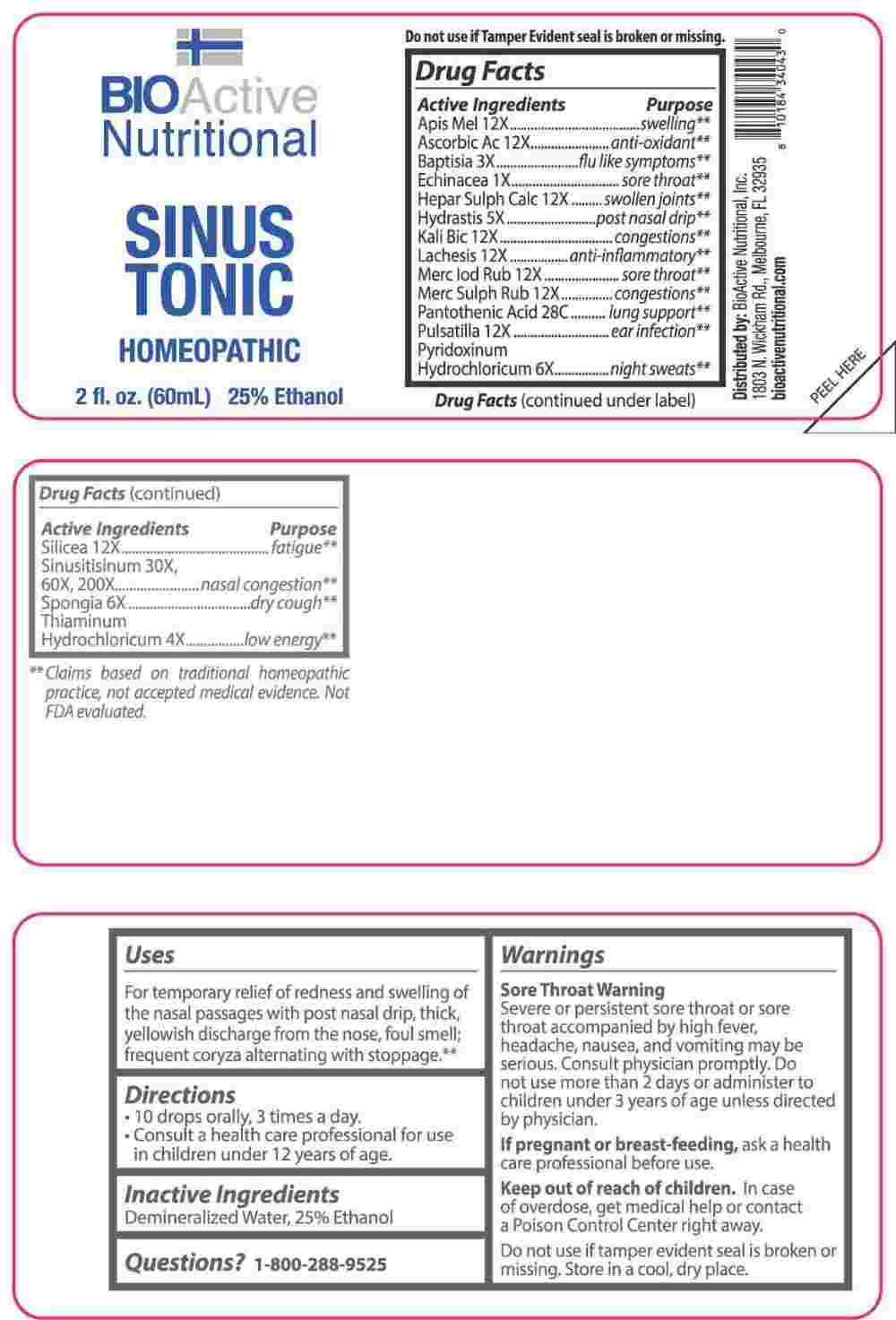
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL DISPLAY:
BIOActiveNutritional
SINUS TONIC
HOMEOPATHIC
2fl. oz. (60mL)
INDICATIONS & USAGE SECTION
USES:
For temporary relief of redness and swelling of the nasal passages with post nasal drip, thick, yellowish discharge from the nose, foul smell; frequent coryza alternating with stoppage.**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
OTC - ACTIVE INGREDIENT SECTION
ACTIVE INGREDIENTS:
Apis Mellifica 12X, Ascorbic Acid 12X, Baptisia Tinctoria 3X, Echinacea 1X, Hepar Sulphuris Calcareum 12X, Hydrastis Canadensis 5X, Kali Bichromicum 12X, Lachesis Mutus 12X, Mercurius Iodatus Ruber 12X, Mercurius Sulphuratus Ruber 12X, Pantothenic Acid 28C, Pulsatilla 12X, Pyridoxinum Hydrochloricum 6X, Silicea 12X, Sinusitisinum 30X, 60X, 200X, Spongia Tosta 6X, Thiaminum, Hydrochloricum 4X.
OTC - PURPOSE SECTION
PURPOSE:
Apis Mellifica - swelling,** Ascorbic Acid – anti-oxidant,** Baptisia Tinctoria – flu like symptoms,** Echinacea – sore throat,** Hepar Sulphuris Calcareum – swollen joints,** Hydrastis Canadensis – post nasal drip,** Kali Bichromicum - congestions,** Lachesis Mutus – anti-inflammatory,** Mercurius Iodatus Ruber – sore throat,** Mercurius Sulphuratus Ruber - congestions,** Pantothenic Acid – lung support,** Pulsatilla – ear infection,** Pyridoxinum Hydrochloricum – night sweats,** Silicea - fatigue,** Sinusitisinum – nasal congestion,** Spongia Tosta – dry cough,** Thiaminum, Hydrochloricum – low energy**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS SECTION
WARNINGS:
Sore Throat Warning
Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult physician promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by physician.
If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
Store in a cool, dry place.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
KEEP OUT OF REACH OF CHILDREN:
In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
DIRECTIONS:
• 10 drops orally, 3 times a day.
• Consult a health care professional for use in children under 12 years of age.
INACTIVE INGREDIENT SECTION
INACTIVE INGREDIENTS:
Demineralized Water, 25% Ethanol
OTC - QUESTIONS SECTION
QUESTIONS:
Distributed by:
BioActive Nutritional, Inc.
1803 N. Wickham Rd.
Melbourne, FL 32935
bioactivenutritional.com
1-800-288-9525
