Milk Fever CP
Milk Fever CP
849cb5d4-3876-47d4-af1d-caf9df557fe9
PRESCRIPTION ANIMAL DRUG LABEL
Sep 9, 2025
Aspen Veterinary
DUNS: 627265361
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Milk Fever CP
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
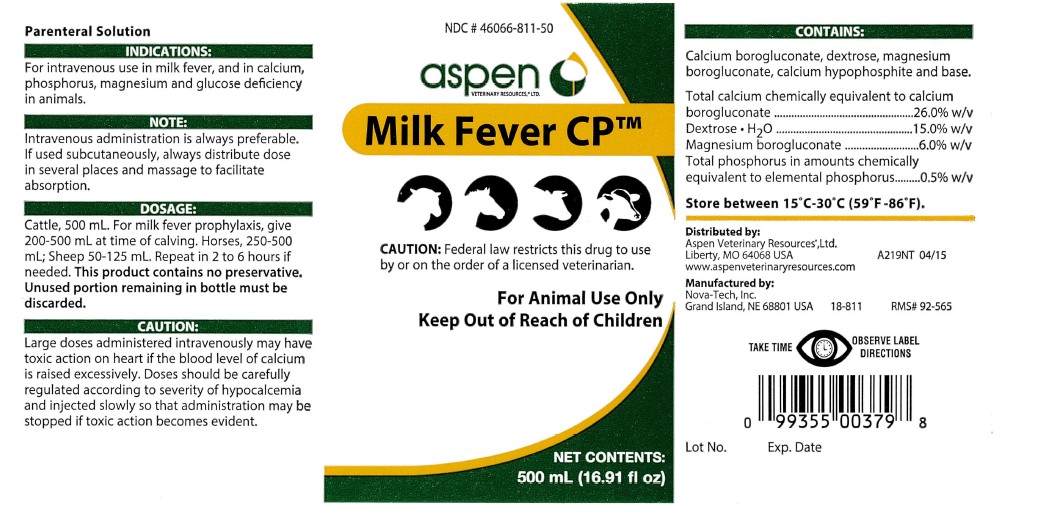
INDICATIONS & USAGE SECTION
NOTE:
Intravenous administration is always preferable.
If used subcutaneously, always distribute dose
in several places and massage to facilitate
absorption.
PRECAUTIONS SECTION
CAUTION:
Federal law restricts this drug to use
by or on the order of a licensed veterinarian.
GENERAL PRECAUTIONS SECTION
TAKE TIME OBSERVE LABEL DIRECTIONS
INFORMATION FOR OWNERS/CAREGIVERS SECTION
Distributed by:
Aspen Veterinary ResourcesR, Ltd.
Liberty, MO 64068 USA
www.aspenveterinaryresources.com
Manufactured by:
Nova-Tech, Inc.
Grand Island, NE 68801 USA 18-811 RMS# 92-565
Lot No. Exp. Date
DOSAGE & ADMINISTRATION SECTION
DOSAGE:
Cattle, 500 mL. For milk fever prophylaxis, give
200-500 mL at time of calving. Horses, 250-500
mL; Sheep 50-125 mL. Repeat in 2 to 6 hours if
needed.This product contains no preservative.
Unused portion remaining in bottle must be
discarded.
DOSAGE FORMS & STRENGTHS SECTION
CONTAINS:
Calcium borogluconate, dextrose, magnesium
borogluconate, calcium hypophosphite and base.
Total calcium chemically equivalent to calcium
borogluconate...................................26.0% w/v
Dextrose . H2O..................................15.0% w/v
Magnesium borogluconate..................6.0% w/v
Total phosphorus in amounts chemically
equivalent to elemental phosphorus.....0.5% w/v
STORAGE AND HANDLING SECTION
Store between 15 degrees C-30 degrees C (59degrees F-86 degrees F).
