Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SUSPENSION
**DOSAGE AND ADMINISTRATION** **Recommended Dose** ADACEL®-POLIO should be administered as a single injection of 1 dose (0.5 mL) by the intramuscular route. The preferred site is the deltoid muscle. ADACEL®-POLIO may be administered to pregnant women during the second or third trimester to provide passive immunization of infants against pertussis (see sections WARNINGS AND PRECAUTIONS – Pregnant Women and ACTION AND CLINICAL PHARMACOLOGY – Immunogenicity – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Fractional doses (doses <0.5 mL) should not be given. The effect of fractional doses on safety and efficacy has not been determined. Health-care professionals should refer to the National Advisory Committee on Immunization (NACI) guidelines for tetanus prophylaxis in routine wound management shown in Table 2. 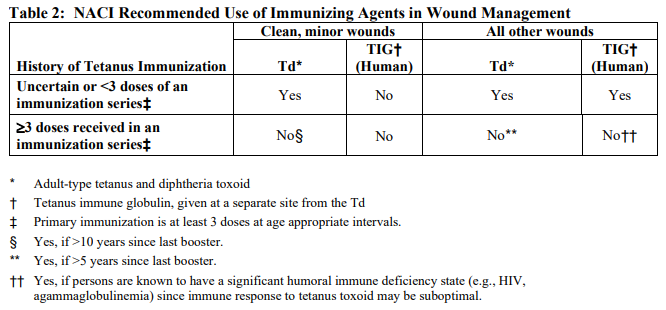 A thorough attempt must be made to determine whether a patient has completed primary immunization. Persons who have completed primary immunization against tetanus and who sustain wounds that are minor and uncontaminated, should receive a booster dose of a tetanus toxoid-containing preparation if they have not received tetanus toxoid within the preceding 10 years. For tetanus-prone wounds (e.g., wounds contaminated with dirt, feces, soil and saliva, puncture wounds, avulsions and wounds resulting from missiles, crushing, burns or frostbite), a booster is appropriate if the patient has not received a tetanus toxoid-containing preparation within the preceding 5 years. For adults who have not previously received a dose of acellular pertussis vaccine, a single Tetanus-diphtheria (Td) booster dose should be replaced by a combined tetanus-diphtheria-acellular pertussis vaccine (Tdap). **Administration** Inspect for extraneous particulate matter and/or discolouration before use. (See DESCRIPTION – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.) If these conditions exist, the product should be discarded. **Shake the vial or syringe well** until a uniform, cloudy, suspension results. When administering a dose from a stoppered vial, do not remove either the stopper or the metal seal holding it in place. Use a separate sterile needle and syringe, or a sterile disposable unit for each individual patient to prevent disease transmission. Needles should not be recapped but should be disposed of according to biohazard waste guidelines. Before injection, the skin over the site to be injected should be cleansed with a suitable germicide. Administer the total volume of 0.5 mL **intramuscularly** (IM). The preferred site of injection is the deltoid muscle.
INTRAMUSCULAR
Medical Information
**INDICATIONS AND CLINICAL USE** ADACEL®-POLIO is indicated for active booster immunization for the prevention of tetanus, diphtheria, pertussis (whooping cough) and poliomyelitis in persons 4 years of age and above. In children 4 to 6 years of age, ADACEL®-POLIO may be considered as an alternative for the fifth dose of diphtheria, tetanus, acellular pertussis and inactivated poliomyelitis vaccine (DTaP-IPV). Persons who have had tetanus, diphtheria or pertussis should still be immunized since these clinical infections do not always confer immunity. Human Immunodeficiency Virus (HIV)-infected persons, both asymptomatic and symptomatic, should be immunized against tetanus, diphtheria and pertussis according to standard schedules. ADACEL®-POLIO is not to be used for the treatment of disease caused by _Bordetella pertussis_, _Corynebacterium diphtheriae_, _Clostridium tetani_ or poliomyelitis infections. **Pediatrics** ADACEL®-POLIO has been used in clinical studies in children as young as 3 years of age. **Geriatrics** ADACEL®-POLIO has been used in clinical studies in persons up to 91 years of age. **Tetanus Prophylaxis in Wound Management** The need for active immunization with a tetanus toxoid-containing preparation (such as Td Adsorbed vaccine, ADACEL® or ADACEL®-POLIO) with or without passive immunization with Tetanus Immune Globulin, depends on both the condition of the wound and the patient’s vaccination history. (See DOSAGE AND ADMINISTRATION.)
**CONTRAINDICATIONS** **Hypersensitivity** Known systemic hypersensitivity reaction to any component of ADACEL®-POLIO or a life-threatening reaction after previous administration of the vaccine or a vaccine containing one or more of the same components are contraindications to vaccination. Because of uncertainty as to which component of the vaccine may be responsible, none of the components should be administered. Alternatively, such persons may be referred to an allergist for evaluation if further immunizations are considered. **Acute Neurological Disorders** Encephalopathy (e.g., coma, decreased level of consciousness, prolonged seizures) within 7 days of a previous dose of a pertussis-containing vaccine that is not attributable to another identifiable cause is a contraindication to administration of any pertussis-containing vaccine, including ADACEL®-POLIO.
J07CA02
diphtheria-pertussis-poliomyelitis-tetanus
Manufacturer Information
SANOFI-AVENTIS SINGAPORE PTE. LTD.
Sanofi Pasteur (VDR)
SANOFI PASTEUR LIMITED
Active Ingredients
Inactivated Poliomyelitis Vaccine (IPV) Type 1 Mahoney
40 D-Antigen Units
(Acellular Pertussis) Filamentous Haemagglutinin Adsorbed (FHA)
5 µg/ 0.5 ml
(Acelullar Pertussis) Pertussis Toxoid Adsorbed (PT)
2.5 µg/ 0.5 ml
Inactivated Poliomyelitis Vaccine (IPV) Type 2 (MEF-1)
8 D-antigen units
Inactivated Poliomyelitis Vaccine (IPV) Type 3 (Saukett)
32 D-antigen units
(Acellular Pertussis) Fimbriae Type 2 and 3 Adsorbed (FIM)
5 µg/0.5 ml
Documents
Package Inserts
Adacel-Polio suspension for injection in a pre-filled syringe PI.pdf
Approved: July 15, 2022
