Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**Dosage and administration** Recommended dosage 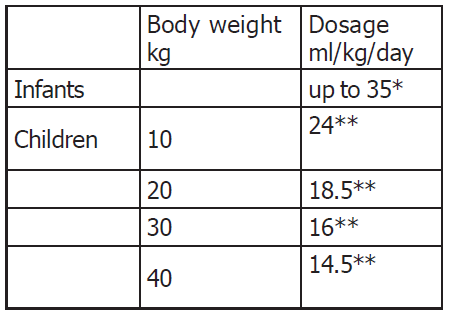 \\* The dosage should be gradually increased to this value during the first week of life and the infusion should preferably be given continuously over 24 hours. \\*\\* The duration of infusion should be at least 8 hours. **Administration** Vaminolact may be infused into the same central of peripheral vein as glucose and fat emulsion by means of a Y-connector near the infusion site. Discard any unused contents.
INTRAVENOUS
Medical Information
**Indications** Vaminolact is indicated as a source of amino acids for protein synthesis and of taurine in infants and children requiring intravenous nutrition.
**Contraindications** Vaminolact is contra-indicated in patients with inborn errors of amino acid metabolism, irreversible liver damage and in severe uremia when dialysis facilities are not available.
B05BA01
amino acids
Manufacturer Information
FRESENIUS KABI (SINGAPORE) PTE LTD
FRESENIUS KABI AUSTRIA GMBH
Active Ingredients
Documents
Package Inserts
Vaminolact Intravenous Solution PI.pdf
Approved: August 4, 2020
