Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
EMULSION
**4.2 Posology and method of administration** **Posology** The dosage depends on the patient’s energy expenditure, clinical status, body weight and the ability to metabolize the constituents of OLICLINOMEL, as well as additional energy intake or proteins taken orally or enterally; therefore the bag size chosen accordingly. The administration may be continued for as long as is required by the patient’s clinical conditions. **Maximum daily dose** The maximum daily dose should not be exceeded in adult and paediatric patients. Due to the static composition of the multi-chamber bag, the ability to simultaneously meet all nutrient needs of the patient may not be possible. Clinical situations may exist where patients require amounts of nutrients varying from the composition of the static bag. _**In adults**_ _Requirements_ Average nitrogen requirements are 0.16 to 0.35g/kg/day (approximately 1 to 2 g of amino acids/kg/day). Energy requirements vary depending on the patient’s nutritional status and level of catabolism. On average these are 20 to 40 kcal/kg/day. _Maximum daily dose_ The maximum daily dose is defined by the energy component. The maximum daily dose is 33 ml/kg body weight (equivalent to 1.32 g amino acids, 5.28 g glucose, 1.32 g lipids, 1.06 mmol sodium and 0.79 mmol potassium / kg), i.e. 2310 ml of the emulsion for infusion for a patient weighing 70 kg. _**In adolescent and children greater than two years of age**_ There have been no studies performed in the paediatric population. Posology: The dosage is based on fluid intake and daily nitrogen requirements. These intakes should be adjusted to take account of the child’s hydration status. Daily fluid, nitrogen, and energy requirements continuously decrease with age. The guidelines for maximal recommended hourly rate of infusion and volume per day for pediatric patients are: **Maximum Daily Dose** 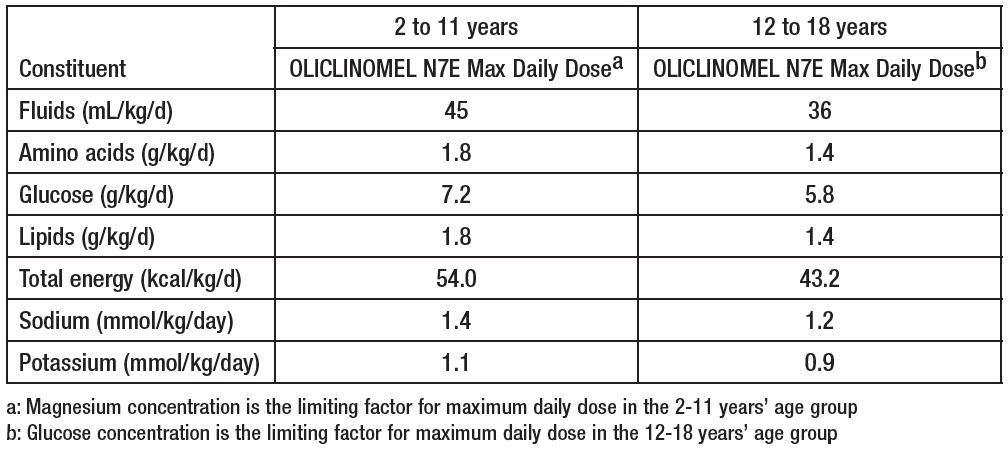 **Maximum Hourly Rate** 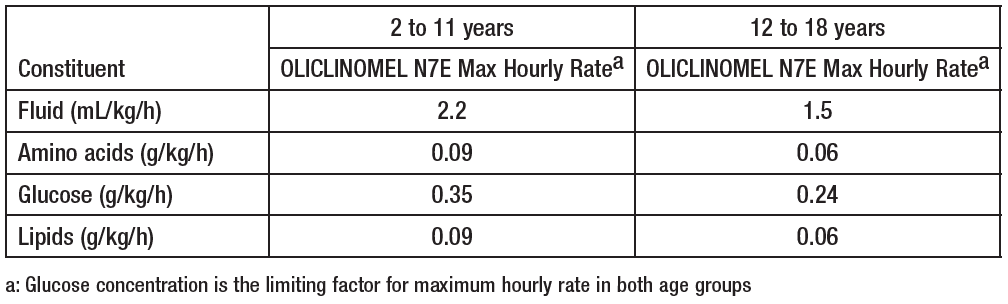 **Method and duration of administration** For single use only. It is recommended that after opening the bag, the contents should be used immediately, and not stored for subsequent infusion. Appearance after reconstitution: homogenous liquid with a milky appearance. For instructions for preparation and handling of the emulsion for infusion, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. BY INTRAVENOUS ADMINISTRATION THROUGH A CENTRAL VEIN ONLY (due to the high osmolarity of OLICLINOMEL). The recommended duration of the parenteral nutrition infusion is between 12 and 24 hours. The administration flow rate must be adjusted taking into account the dose being administered, the daily volume intake, and the duration of the infusion (See Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Normally, the flow rate should be increased gradually during the first hour. _Maximum infusion rate in adults:_ As a general rule, do not exceed 1.5 ml/kg/hour of the emulsion for infusion, i.e. 0.06 g amino acids, 0.24 g glucose and 0.06 g lipids /kg body weight / hour. As a general rule, do not exceed infusion rates of 0.10 g/kg/hour amino acids and/or 0.25 g/kg/hour glucose and/or 0.15 g/kg/hour lipids, except in particular cases.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Parenteral nutrition for adults and children greater than two years of age, when oral or enteral nutrition is impossible, insufficient or contraindicated.
**4.3 Contraindications** The use of OLICLINOMEL N7-1000E is contraindicated in the following situations: - In premature neonates, infants and children less than 2 years old, as the calorie‐nitrogen ratio and energy supply are inappropriate; - Hypersensitivity to egg, soya-bean, peanut proteins, or corn/corn products (see Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_), or to any other active substance or excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_; - Congenital abnormalities of amino acid metabolism; - Severe hyperlipidaemia or severe disorders of lipid metabolism characterized by hypertriglyceridaemia; - Severe hyperglycaemia; - Pathologically elevated plasma concentrations of sodium, potassium, magnesium, calcium and/or phosphorus.
B05BA10
combinations
Manufacturer Information
BAXTER HEALTHCARE (ASIA) PTE LTD
BAXTER SA
Ajinomoto North America Incorporated (Intermediate)
Active Ingredients
REFINED SOYA BEAN OIL + REFINED OLIVE OIL (In Compartment 1)
40.00 g/l
Documents
Package Inserts
OliClinomel N7-1000E PI.pdf
Approved: August 6, 2021
